Abstract
Glioma is one of the most common primary brain tumors. Despite surgical resection, radiotherapy, and chemotherapy, the prognosis of patients with malignant glioma remains poor. Programmed cell death 5 (PDCD5) is a newly described pro-apoptotic protein. Our previous study showed that PDCD5 downregulation in gliomas was associated with higher pathological grade. Here, we investigated the effect of PDCD5 on chemosensitivity of glioma cells and its mechanism. We demonstrated that overexpression or knockdown of PDCD5 had no significant effect on the proliferation of glioma cell lines (U87, U251, and T98G) in the absence of chemotherapeutic agents. However, PDCD5 overexpression effectively sensitized U87 cells to chemotherapeutic drugs (cisplatin, carboplatin, and vincristine) in a concentration-dependent manner, while its knockdown resulted in decreased chemosensitivity in U251, T98G, and U87 cells. Importantly, expression of PDCD5 also markedly inhibited tumor cell proliferation and colony formation in the presence of low doses of cisplatin. Furthermore, we found that PDCD5 expression promoted cisplatin-induced apoptosis, increased markedly the activation of caspase-3 and caspase-9, and decreased significantly the ratio of Bcl-2/Bax proteins, but had no effect on the activation of caspase-8. Taken together, our findings indicate that PDCD5 promotes chemosensitivity by activating mitochondria-related apoptotic pathway, and that the combination of PDCD5 and chemotherapeutic drugs such as cisplatin, is expected to be an effective therapeutic strategy for the malignant glioma.
Keywords: :
Introduction
Programmed cell death 5 (PDCD5), also called TF-1 cell apoptosis-related gene-19 (TFAR19), is a strong candidate of apoptosis-regulated protein.Citation1 PDCD5 protein is upregulated in the process of cell apoptosis and transports rapidly from the cytoplasm to the nucleus.Citation2 When PDCD5 is transiently or stably overexpressed in tumor cell lines such as TF-1 and HL-60 cells, PDCD5 promotes apoptosis triggered by certain stimuli rather than initiates apoptotic occurrence.Citation1,Citation3 However, blocking PDCD5 action by anti-PDCD5 antibody suppresses apoptosis induced by etoposideCitation4 and knocking down its expression by PDCD5-specific siRNA attenuates cell apoptosis induced by Bax overexpression.Citation5 The phosphorylation of PDCD5 by CK2, a multifunctional kinase, is an important process for its apoptotic potential.Citation6 The interaction of PDCD5 with Tip60, a histone acetyltransferase, accelerates DNA damage-induced apoptosis while knockdown of PDCD5 or Tip60 impairs their apoptosis-accelerating activity.Citation7
Recent studies have demonstrated that PDCD5 is downregulated in multiple kinds of human tumors such as gastric cancer,Citation8 hepatocellular carcinoma,Citation9 breast cancer,Citation10 and acute and chronic myeloid leukemia.Citation11 The reduced expression of PDCD5 correlates with short survival periods of patients.Citation8,Citation12-Citation14 Furthermore, it has been found that two single-nucleotide polymorphisms with linkage disequilibrium in the 5′-regulatory region of human PDCD5 gene reduce its promoter activity and increase susceptibility to chronic myeloid leukemia.Citation15,Citation16 A single-nucleotide polymorphism in the 5′-upstream region of PDCD5 gene is predictive for lung cancer risk and prognosis.Citation17 In addition, exogenous PDCD5 protein, or adenovirus carrying PDCD5 gene, exerts potent antitumor efficacy on human leukemic cells in vitro and in vivo.Citation18 Our previous research demonstrated that PDCD5 expression reduced markedly in gliomas compared with brain tissue adjacent to the tumor and its decreased expression correlated significantly with high-grade astrocytoma.Citation19 These data suggest that PDCD5 may represent a novel tumor suppressor gene which influences various kinds of cancers including glioma.
Human glioma is the most common type of primary brain tumors. Standard treatment for patients with glioma is surgery combined with radiotherapy and chemotherapy. Although chemotherapy has showed a little improvement in survival period of patients with glioma by meta-analysis, the effectiveness is not satisfactory due to its severe side effects of drugs and chemoresistance.Citation20 Therefore, it is necessary to explore novel approaches that can improve chemosensitivity of tumors. In the present study, we have investigated the effect of PDCD5 expression on the sensitivity of glioma cells to chemotherapeutic drugs, cisplatin (CDDP), carboplatin (CBDCA), vincristine (VCR), etoposide (VP-16), and then explored the mechanism by which PDCD5 sensitized glioma cells to CDDP. We demonstrated that PDCD5 overexpression is able to enhance the chemosensitivity of glioma cells to CDDP via mitochondria-related apoptotic pathway.
Results
Exogenous expression of PDCD5 had no significant effect on the proliferation of glioma cells
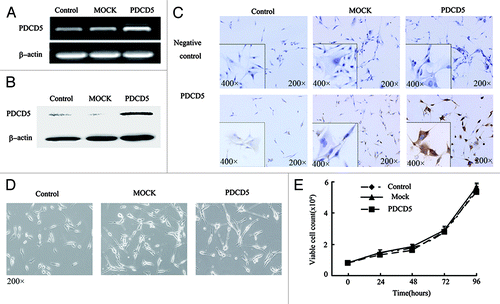
To investigate the potential role of PDCD5 in glioma, we overexpressed PDCD5 in U87 cells, which expressed a low level of PDCD5. The increased expression of PDCD5 at mRNA and protein levels was detected in U87-PDCD5 cells compared with control U87 cells, and U87-MOCK cells by RT-PCR (), western blot (), and immunocytochemistry (). However, PDCD5 overexpression had no significant impact on the morphology and proliferation of glioma cells in the absence of apoptotic inducer (p > 0.05) ().
Figure 1. The stable expression of exogenous PDCD5 in U87 cells and its effect on cell morphology and proliferation. The expression of PDCD5 in U87 cells was detected by RT-PCR (A), western blot (B) and immunocytochemistry (C) after stably transfected with pcDNA3.1(MOCK) or pcDNA3.1-PDCD5 (PDCD5). PDCD5 expression was significantly increased in PDCD5-transfected cells compared with corresponding control cells. (D) The morphology of U87 cells stably transfected with MOCK (U87-MOCK) or PDCD5 plasmid (U87-PDCD5). (E) The effect of exogenous expression of PDCD5 on the growth of U87 cells.
Overexpression of PDCD5 sensitized selectively glioma cells to chemotherapeutic drugs
To investigate the effect of PDCD5 expression on the chemosensitivity of glioma cells, U87-MOCK and U87-PDCD5 cells were treated with CDDP, CBDCA, VCR, and VP-16 which have been used widely in the treatment of malignant gliomas. As shown in , the growth of U87-PDCD5 cells with high levels of PDCD5 expression was slower than that of U87-MOCK cells after treatment with the same concentration of CDDP. The viability of cells decreased from 70.52% of U87-MOCK to 33.63% of U87-PDCD5 cells in presence of 12.5 μg/ml of CDDP, which implied that U87 cells highly expressing PDCD5 were more sensitive to CDDP (). Similarly, overexpression of PDCD5 also increased sensitivity of U87 cells to CBDCA and VCR (). However, it had no significant effect on sensitivity to VP-16 (). The effect of PDCD5 overxpression on IC50 values for CDDP, CBDCA, VCR, and VP-16 was listed in .
Figure 2. The effect of PDCD5 expression on the chemosensitivity of glioma cells. (A) The morphology changes of U87 cells transfected with MOCK or PDCD5 plasmid treated with different concentrations of CDDP for 48h. U87-PDCD5 cells became round after treatment with 12.5 μg/ml CDDP, while U87-MOCK cells change under the action of 25 μg/ml CDDP. (B–E) The survival of U87-MOCK and U87-PDCD5 cells was examined by MTT assay when they were exposed to CDDP, CBDCA, VCR, and VP-16 for 48 h. Values are the mean ± SEM, all the experiments were repeated three times. Overexpression of PDCD5 increased sensitivity of U87 cells to CDDP, CBDCA and VCR but had no effect on VP-16 (*p < 0.05, **p < 0.01).
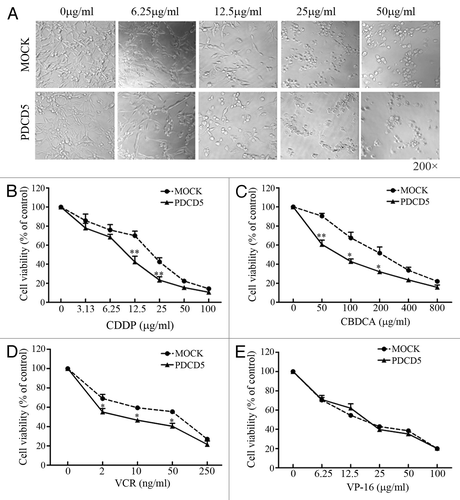
Table 1. The effect of PDCD5 overexpression on IC50 values of chemotherapeutic drugs
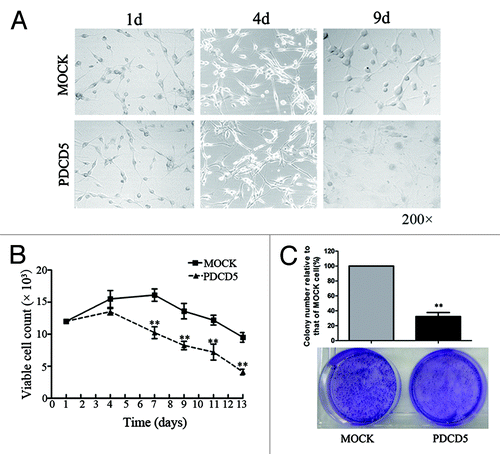
Since high doses of chemotherapeutic drugs often cause the severe side effects in cancer patients, the long-term application of drugs is limited in clinic. To investigate whether PDCD5 expression also enhances chemosensitivity of tumor cells to low doses of drugs, we treated U87-MOCK and U87-PDCD5 cells with 3μg/ml CDDP. As shown in , morphology of U87-PDCD5 cells had no significant difference compared with that of U87-MOCK cells within four days in the presence of 3 μg/ml CDDP. However, 4 d later, number of viable cells in U87-PDCD5 group markedly decreased compared with that in U87-MOCK group (). Moreover, the capacity of colony formation of U87-PDCD5 cells significantly reduced compared with that of U87-MOCK cells in presence of the low concentration of CDDP (). These data indicate that PDCD5 overexpression can enhance chemosensitivity of tumor cells to low doses of drugs.
Figure 3. The effect of exogenous expression of PDCD5 on the growth of glioma cells treated with low doses of chemotherapeutic agents. (A) The morphology of U87-MOCK and U87-PDCD5 cells when treated with low concentration of CDDP (3 μg/ml) for 1, 4 and 9 d. (B) The cell viability of U87-MOCK and U87-PDCD5 cells when treated with low concentration of CDDP. One week later, number of viable cells in U87-PDCD5 group markedly decreased compared with that in U87-MOCK group (**p < 0.01). (C) The colony formation of U87-MOCK and U87-PDCD5 cells when treated with low concentration of CDDP (3 μg/ml) for 2 weeks. The colony-forming capacity of U87-PDCD5 cells was significantly reduced compared with U87-MOCK cells. Data are one representation of three separate experiments.
The knockdown of PDCD5 decreased chemosensitivity of glioma cells to CDDP
To further confirm the effect of PDCD5 on chemosensitivity, we knocked down PDCD5 expression in U87-PDCD5, U251, and T98G cells using PDCD5-specific siRNAs (siPDCD5-1, -2), non-targeting siRNA as negative control. Forty-eight hours after transfection, both PDCD5 mRNA and protein were significantly decreased in U87-PDCD5, U251, and T98G cells by RT-PCR and western blot (). siPDCD5-2 was more efficient and expression of PDCD5 in U87 cells was decreased by 60.47% at mRNA level and by 66.14% at protein level. Therefore, siPDCD5-2 was used in the following experiments. Similar to PDCD5 overexpression, the knockdown of PDCD5 expression had no significant effect on morphology and on the proliferation rate of giloma cells in the absence of apoptotic inducer (data not shown). However, in the presence of CDDP, knockdown of PDCD5 in U87-PDCD5, U251 cells significantly attenuated the sensitivity of cells to CDDP () and reduced IC50 values (). Otherwise, knockdown of PDCD5 in T98G only depressed sensitivity of cells to CDDP at 25 μg/ml (), but had no significant effect on IC50 values (), suggesting that the effect of PDCD5 on chemosensitivity may hae cell type specificity.
Figure 4. The effect of PDCD5 knockdown on the chemosensitivity of glioma cells to CDDP. U87-PDCD5(A), U251(B) and T98G (C) cells were transfected with 5 nM siRNA-1 (P-1), siRNA-2 (P-2) and negative control siRNA (non-specific oligonucleotides, NC), 48 h after transfection, the expression of PDCD5 were detected by RT-PCR and western blot. PDCD5 expression was significantly decreased in cells transfected with siRNA-2 (P-2). The chemosensitivity of U87-PDCD5 (D), U251 (E) and T98G (F) cells to CDDP after knockdown of PDCD5 by siRNA. Knockdown of PDCD5 significantly decreased sensitivity of U87-PDCD5 cells and U251 cells to CDDP, and slightly in T98G cells. Data are expressed as the mean of triplicates ± SEM (*p < 0.05, **p < 0.01).
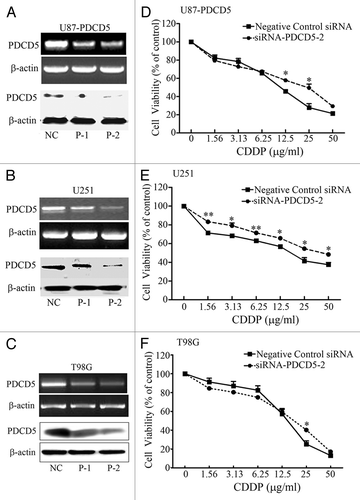
Table 2. The effect of PDCD5 silence in three glioma cell lines on IC50 values of CDDP
The enforced expression of PDCD5 promoted CDDP-induced apoptosis in glioma cells
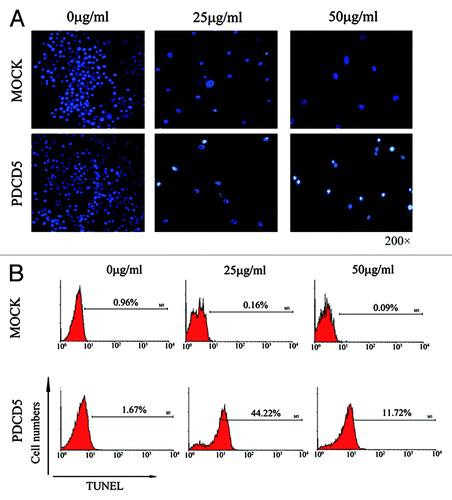
To further determine the mechanism of PDCD5 enhancing chemosensitivity of glioma cells, we analyzed the apoptosis of U87-MOCK and U87-PDCD5 cells treated with CDDP. Hoechst staining revealed those U87-PDCD5 cells appeared as conspicuous nuclear condensation and underwent more apoptosis than U87-MOCK cells in the presence of CDDP (). Furthermore, the results of TUNEL assay also clearly showed more apoptotic cells in U87-PDCD5 group (44.22%) than U87-MOCK group (0.16%) at 25μg/ml CDDP. Similar results were achieved at 50 μg/ml of CDDP (). All these results indicate that PDCD5 can enhance chemosensitivity to CDDP by promoting CDDP-induced apoptosis.
Figure 5. The effect of exogenous expression of PDCD5 on CDDP-induced apoptosis in glioma cells. (A) The apoptosis of U87-MOCK and U87-PDCD5 cells when treated with different concentrations of CDDP for 48 h (Hoechst, 200× ). U87-PDCD5 cells appeared conspicuous nuclear condensation and underwent more apoptosis than U87-MOCK cells in the present of CDDP. (B) The late apoptosis of U87-MOCK and U87-PDCD5 cells after treated with CDDP for 48 h by TUNEL assay. U87-PDCD5 cells showed more apoptosis than U87- MOCK cells at 25 μg/ml CDDP.
PDCD5 expression enhanced CDDP-induced apoptosis via mitochondrial pathway
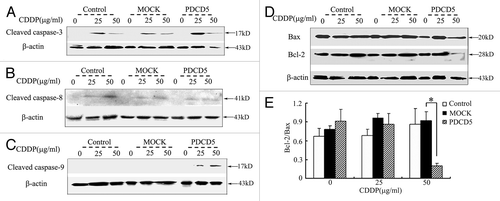
To determine the pathway by which PDCD5 promoted CDDP-induced apoptosis, we detected the expression of apoptosis-related proteins, such as cleaved caspase-3, cleaved caspase-8, cleaved caspase-9, Bcl-2, and Bax. Compared with the controls, we found that PDCD5 overexpression increased cleavage of caspase-3 and caspase-9, but had no significant influence on that of caspase-8 expression after treatment with CDDP for 48 h (). Furthermore, we found that the ratio of anti-apoptotic Bcl-2 and pro-apoptotic Bax (Bcl-2/Bax) in U87-PDCD5 cells decreased after 50 μg/ml CDDP treatment (). Taken together, our results indicate that PDCD5 can enhance CDDP-induced apoptosis by activating mitochondrial apoptotic pathway.
Figure 6. The effect of exogenous overexpression of PDCD5 on apoptosis related proteins. (A) The expression level of cleaved caspase-3 was examined by western blot. Expression of cleaved caspase-3 was significantly increased in U87-PDCD5 cells than those of two control cells (Control and MOCK groups) after treatment with CDDP for 48 h. (B) The expression level of cleaved caspase-8 was examined by western blot. There was no significant difference about cleaved caspase-8 expression between U87-PDCD5 cells and control cells after CDDP treatment. (C) The expression level of cleaved caspase-9 was examined by western blot. Expression of cleaved caspase-9 was significantly increased in U87-PDCD5 cells than those of two control cells after treatment with CDDP for 48 h. (D) The protein of Bcl-2 and Bax in U87-MOCK and U87-PDCD5 cells after treated with CDDP (0, 25, 50μg/ml) for 48 h was examined by western blot. (E) The expression of Bcl-2 and Bax was analyzed by normalized to β-actin. The ratio of Bcl-2/Bax in U87-PDCD5 cells decreased after 50 μg/ml CDDP treatment (*p < 0.05). Control: untransfected cells; MOCK: transfected cells with plasmids pcDNA3.1; PDCD5: transfected cells with plasmids pcDNA3.1 containing full length of PDCD5 gene.
Discussion
In the present study, we demonstrate that PDCD5 is able to enhance selectively sensitivity of glioma cells to CDDP, CBDCA, VCR but not VP-16. Furthermore, our data indicate that PDCD5 promotes CDDP-induced apoptosis via activating the mitochondrial apoptotic pathway.
Our previous studies showed that PDCD5 expression is downregulated in human glioma. However, the effect of PDCD5 on the chemosensitivity and its mechanism remain to be uninvestigated. CDDP, CBDCA, VCR, and VP-16 are the most common chemotherapeutic drugs in the treatment of malignant gliomas. In this study, we demonstrated that PDCD5 expression level affects the sensitivity of glioma cells to these common drugs in vitro. Overexpression of PDCD5 in U87 cells significantly increases sensitivity of tumor cells to CDDP, while knockdown of PDCD5 decreases that to the same drug. The results are in accordance with previous reports which showed that PDCD5 sensitizes chondrosarcomas or colorectal cancer to cisplatin chemotherapy.Citation21,Citation22 In addition, we found that PDCD5 also increased chemosensitivity to CBDCA and VCR. However, PDCD5 had no significance effect on sensitivity of glioma cells to VP16. This result is inconsistent with one previous report, which showed that PDCD5 enhanced the apoptosis of K562 cells induced by VP-16.Citation23 A possible explanation for the discrepancies in different types of human cancers is that the effect of PDCD5 on the chemosesitivity may have cell type specificity. Since PDCD5 is a pro-apoptotic molecule, its action may depend on other apoptotic proteins. We have found that the effect of PDCD5 on chemosensitivity relates not only to PDCD5, but also to other apoptosis-related protens, such as p53 and PDCD4 (data not shown).
The high doses of chemotherapeutic drugs often cause severe side effects, while low doses of drugs are easy to induce the chemoresistance in clinic. Therefore, to explore new methods that sensitize tumor cells to low doses of drugs is very important for the improvement of survival period of patients with tumors. In the present research, we found that overexpression of PDCD5 in combination with low doses of CDDP not only markedly attenuated proliferation of tumor cells, but also inhibited the capacity of colony formation, indicating that PDCD5 overexpression can enhance the sensitivity of tumor cells to low doses of drugs. This will be more helpful for treatment of cancer patients. It has been reported that adenovirus carrying PDCD5 possesses a potent antitumor effect,Citation21,Citation24 and exogenous human PDCD5 protein has also antitumor activities.Citation22,Citation25 Thus, combination of PDCD5 protein or gene with chemotherapeutic drugs may be a new strategy for tumor treatment including glioma.
CDDP is a conventional chemotherapeutic drug for the treatment of malignant tumors. One of the mechanisms is to induce apoptosis of tumor cells via the mitochondrial pathway and death receptor pathway (non-mitochondrial pathway).Citation26,Citation27 The death receptor pathway activates initiator caspase-8 and then cleaves its downstream effector caspases, such as caspase-3 which transducts the apoptosis signal, while the mitochondrial pathway is initiated by the mitochondrial dysfunction and then causes activation of caspase-9 which triggers caspase-3 activation.Citation28 In addition, the balance of proapoptotic and antiapoptotic protein such as Bcl-2 family members regulates the sensitivity of cells to apoptosis by affecting mitochondrial function. Bax and Bcl-2 are important proapoptotic and antiapoptotic proteins in Bcl-2 family, respectively.Citation28 The decreased ratio of Bcl-2/Bax causes the mitochondrial dysfunction with alterations in the permeability transition pore, the release of cytochrome c, and then activation of caspase-9.Citation29 In this study, we detected the effect of PDCD5 overexpression on CDDP-induced apoptosis. The results showed that overexpression of PDCD5 could promote CDDP-induced apoptosis. Noteworthy, the percentage of apoptotic cells in presence of 25 µg/ml of CDDP was higher than that in presence of 50 µg/ml of CDDP detected by flow cytometry (). This was accordance with the expression of cleaved caspase-3 detected by western blot in but was inconsistent to death curves in , which showed that death cells in presence of 50 µg/ml of CDDP was higher than that in presence of 25 µg/ml of CDDP. The possible explanation is that 50 µg/ml of CDDP could induces more apoptotic cells to death, which may cause more death cells in death curves but less apoptotic cell in TUNEL staining and detection of cleaved caspase 3 expression. However, we could not eliminate other possibilities. Moreover, we found that PDCD5 increased the cleaved caspase-9 expression in the presence of 25 µg/ml or 50µg/ml CDDP. The ratio of Bax/Bcl-2 obviously decreased in the presence of 50 µg/ml CDDP, while there was no obvious change in the presence of 25 µg/ml CDDP. This indicates that the change of Bax/Bcl-2 may be not the cause of activation of caspase-9. The other mechanisms need to be explored in future. However, our result showed PDCD5 expression had no effect on activation of caspase-8. Caspase-9 is the most important molecule in the downstream of mitochondrial apoptotic pathway, so the change of the cleaved caspase-9 expression indicated that PDCD5 could sensitize glioma cells to chemotherapeutic drugs via promoting mitochondrial apoptotic pathway. This conclusion is consistent with previous reports in chondrosarcomaCitation21 and colorectal cancer.Citation22 Taken together, previous research and our data indicate that PDCD5 promotes chemosensitivity of malignant tumors by activating mitochondrial pathway. Therefore, combination of PDCD5 with chemotherapeutic agents is expected to be a very effective strategy for the treatment of tumors.
Materials and Methods
Cell culture and transfection
U87, U251, and T98G cell lines derived from human primary gliomas were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences. They were cultured and maintained in Dulbecco's modified Eagle's medium (DMEM) (Gibco-BRL, 31600-026) containing 10% fetal bovine serum (Gibco-BRL, 16250-078). Transfection of U87 cells with plasmids pcDNA3.1 and pcDNA3.1-PDCD5 that were presented by Dr Dalong Ma (The Center for Human Disease Genomics, Peking University) was performed using Lipofectamine 2000 according to the manufacturer’s protocols (Invitrogen, 11668-027). To acquire stably-transfected cell clones, pcDNA3.1 or pcDNA3.1-PDCD5-transfected U87 cells were selected in cultured medium containing 450 μg/mL G418 after 24 h of transfection. Several weeks later, the G418-resistant cell clones, designated as U87-MOCK and U87-PDCD5, were collected. In order to verify effectiveness of transfection, RT-PCR, western blot and immunocytochemistry were used for the detection of PDCD5 expression.
RNA isolation and semi-quantitative RT-PCR
Total RNA was extracted using a modified TRIzol one-step extraction method (Invitrogen Corp., 15596-026). cDNA was synthesized using the Reverse-Transcribe kit (Promega Co., A3801) according to manufacturer's instructions. The reaction was performed at 70 þC for 10 min and then at 42 þC for 1 h, followed by 95–99 þC for 5 min to stop the reaction. PCR was performed using PDCD5 specific primers (sense 5′-CCA TGG CGG ACG AGG AGC TTG-3′, and anti-sense 5′-TCA ATA ATC GTC ATC TTC ATC-3′) for 30 cycles at 94 þC for 30 sec, 58 þC for 30 sec and 72 þC for 30 sec followed by an extension cycle at 72 þC for 7 min.
SDS-PAGE and western blot
Rabbit polyclonal antibodies against Bax (Cell Signal Technology, Inc., 2772), Bcl-2 (2872), cleaved caspase-3 (9661s), -9 (9501s), and rabbit monoclonal antibody to cleaved caspase-8 (9496s). Rabbit anti-human β-actin polyclonal antibody was purchased from Santa Cruz Biotechnology, Inc. (3597s). Mouse anti-human PDCD5 monoclonal antibody was presented by Dr Dalong Ma (The Center for Human Disease Genomics, Peking University). The proteins were extracted from glioma cells using a modified TRIzol one-step extraction method. The concentration of the protein was determined by Bradford analysis. The protein extract was dissolved in a loading buffer (1 mM Tris-Cl, 3% SDS, 60% glycerol and 75 mM DTT) and each sample (50 μg) was analyzed by SDS-PAGE on a 15% gel and then transfered onto PVDF membrane. The protein on PVDF membrane was incubated with antibodies at 4 þC overnight followed by secondary antibodies (goat anti-rabbit IgG, Santa Cruz, 95573) conjugated with peroxidase for 1h at room temperature. After washing, signals were visualized by SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology, 34080). Western blot was performed at least three times for each sample.
Immunocytochemistry
U87,U87-MOCK,U87-PDCD5 cells were cultured in 24-well plates treated with Poly-l-lysine for 48h and then fixed by 4% paraformaldehyde for 15 min, stained with rabbit anti-human PDCD5 antibody for 1 h at room temperature. Secondary staining with HRP-conjugated anti-rabbit IgG was performed using a MaxVision kit (Maixin Co., KIT-5004) and a DAB Peroxidase Substrate kit (DAB-0031). Negative control for the specificity of immunocytochemistry reactions was performed by replacing the primary antibody with IgG of non-immunized rabbit.
MTT assay
CDDP, CBDCA, VCR, and VP-16 were obtained from QiLu Pharmaceutical Manufacture. Glioma cells (8 × 103 cells/well) were seeded in 96-well plates. After 24 h of incubation, cells were treated with various concentrations of drugs. After 48 h of treatment, 20 μL of 5 mg⁄mL MTT reagents (Sigma-Aldrich, M2128) was added and incubated another 4 h. Then the absorbance was measured at 570 nM. The cell viability was calculated by the following formula: the absorbance of treated cells/ that of the untreated cell × 100%. This experiment was repeated three times.
Colony formation assay
Stable cell lines U87-MOCK and U87-PDCD5 were cultured in 6-well plates at a density of 103 cells/well in the presence of G418 (450 μg/ml) and CDDP (3 μg/ml) for 2 weeks. The cells were fixed with 20% methanol and stained with 1% crystal violet. Colonies which are composed of more than 50 cells were counted and calculated as a percentage of that of the control group (U87-MOCK). Each cell line was repeated in two wells and the experiment was repeated three times.
Synthesis and transfection of PDCD5 siRNAs
Two different Silencer Select Pre-designed siRNAs targeting PDCD5 and Negative control siRNA (non-specific oligonucleotides, has no significant homology to any known gene sequences from mouse, rat, human) were purchased from Ambion (Ambion Inc., AM4511). siPDCD5–1: sense 5′AGAAUUACCUUAUACAGA3′; antisense 5′UCUGUAAGGUAAUUCUCTA3′. siPDCD5–2: sense 5′GAACAAGGUUUAAUA GAAA3′; antisense 5′UUUCUAUUAAACCUUGUUCTG3′. Cells (5 × 103 cells/well) were seeded in 96-well plates and incubated at standard conditions for 12 h. The siRNAs were transfected into cells using siPORT NeoFX Transfection Agent (Ambion Inc.) according to the manufacturer’s protocol. Forty-eight hours after transfection, cells were treated with a series of concentrations of CDDPs, and the cell viability was determined by MTT assay after 48 h.
Measurement of cell apoptosis
Apoptosis was determined by Hoechst 33258 staining (Nanjing KeyGen Biotech. Co. Ltd, KGA218) or TUNEL assay (Roche, 11 684 795 910) Cells (1 × 104 cells/well) were plated in a culture slide and allowed to adhere to the slide overnight. Then CDDP (25 or 50 μg/ml) was added, the cells were incubated for an additional 48 h and fixed with 4% paraformaldehyde for 20 min. Hoechst 33258 staining was performed in these cells for 20 min and the cells were washed three times with distilled water. Finally, they were viewed under a fluorescence microscope (Leica). Chromatin condensation, nuclear shrinkage, and nucleosomal fragmentation were considered morphologic markers of apoptosis. For TUNEL assay, cells (5 × 105 cells/well) were seeded in 6-well plates and treated with CDDP (25 or 50 μg/ml) for 48 h. Apoptotic cells were labeled using an in situ apoptosis detection kit (Immunotech) by TdT (terminal deoxynucleotidyl transferase)-mediated dUTP nick end-labeling (TUNEL). The degree of apoptosis was determined by ñow cytometry (Beckman Coulter FC500).
Data analysis
All data were expressed as means ± SEM. Statistical evaluations were achieved by one-way analysis of variance and Student’s t-test. p < 0.05 was considered to be significant.
Acknowledgments
This work was supported partially by the National Natural Science Foundation of China (81072407, 81172863), National “973” program of China (2011CB503906), Shandong Province foundation for Medical Pioneer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Liu H, Wang Y, Zhang Y, Song Q, Di C, Chen G, et al. TFAR19, a novel apoptosis-related gene cloned from human leukemia cell line TF-1, could enhance apoptosis of some tumor cells induced by growth factor withdrawal. Biochem Biophys Res Commun 1999; 254:203 - 10; http://dx.doi.org/10.1006/bbrc.1998.9893; PMID: 9920759
- Chen Y, Sun R, Han W, Zhang Y, Song Q, Di C, et al. Nuclear translocation of PDCD5 (TFAR19): an early signal for apoptosis?. FEBS Lett 2001; 509:191 - 6; http://dx.doi.org/10.1016/S0014-5793(01)03062-9; PMID: 11741587
- Zhang YM, Liu HT, Song QS, Ma DL. The apoptosis-accelerating effect of human recombinant TFAR19 protein on leukemia HL-60 cells. J Clin Immunol 2000; 16:8 - 11
- Rui M, Chen Y, Zhang Y, Ma D. Transfer of anti-TFAR19 monoclonal antibody into HeLa cells by in situ electroporation can inhibit the apoptosis. Life Sci 2002; 71:1771 - 8; http://dx.doi.org/10.1016/S0024-3205(02)01943-4; PMID: 12151055
- Chen LN, Wang Y, Ma DL, Chen YY. Short interfering RNA against the PDCD5 attenuates cell apoptosis and caspase-3 activity induced by Bax overexpression. Apoptosis 2006; 11:101 - 11; http://dx.doi.org/10.1007/s10495-005-3134-y; PMID: 16374546
- Salvi M, Xu D, Chen Y, Cabrelle A, Sarno S, Pinna LA. Programmed cell death protein 5 (PDCD5) is phosphorylated by CK2 in vitro and in 293T cells. Biochem Biophys Res Commun 2009; 387:606 - 10; http://dx.doi.org/10.1016/j.bbrc.2009.07.067; PMID: 19616514
- Xu L, Chen Y, Song Q, Xu D, Wang Y, Ma D. PDCD5 interacts with Tip60 and functions as a cooperator in acetyltransferase activity and DNA damage-induced apoptosis. Neoplasia 2009; 11:345 - 54; PMID: 19308289
- Yang YH, Zhao M, Li WM, Lu YY, Chen YY, Kang B, et al. Expression of programmed cell death 5 gene involves in regulation of apoptosis in gastric tumor cells. Apoptosis 2006; 11:993 - 1001; http://dx.doi.org/10.1007/s10495-006-6714-6; PMID: 16547588
- Xu XR, Huang J, Xu ZG, Qian BZ, Zhu ZD, Yan Q, et al. Insight into hepatocellular carcinogenesis at transcriptome level by comparing gene expression profiles of hepatocellular carcinoma with those of corresponding noncancerous liver. Proc Natl Acad Sci U S A 2001; 98:15089 - 94; http://dx.doi.org/10.1073/pnas.241522398; PMID: 11752456
- Duggan D, Chen Y, Radmacher M, Bittner M, Simon R, et al. Gene-expression profiles in hereditary breast cancer. N Engl J Med 2001; 344:539 - 48; http://dx.doi.org/10.1056/NEJM200102223440801; PMID: 11207349
- Ruan GR, Qin YZ, Chen SS, Li JL, Ma X, Chang Y, et al. Abnormal expression of the programmed cell death 5 gene in acute and chronic myeloid leukemia. Leuk Res 2006; 30:1159 - 65; http://dx.doi.org/10.1016/j.leukres.2005.12.028; PMID: 16507320
- Jiang TB, Li X, Zhou J, Zhou Y, Yuan H, Xiang H, et al. [Expression of PDCD5 in multiple myeloma and its relation with BCL-2]. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2008; 33:814 - 20; PMID: 18812660
- Zhang X, Wang X, Song X, Wei Z, Zhou C, Zhu F, et al. Clinical and prognostic significance of lost or decreased PDCD5 expression in human epithelial ovarian carcinomas. Oncol Rep 2011; 25:353 - 8; http://dx.doi.org/10.3892/or.2010.1103; PMID: 21165576
- Chen C, Zhou H, Xu L, Liu X, Liu Z, Ma D, et al. Prognostic significance of downregulated expression of programmed cell death 5 in chondrosarcoma. Prognostic significance of downregulated expression of programmed cell death 5 in chondrosarcoma. J Surg Oncol 2010; 102:838 - 43; http://dx.doi.org/10.1002/jso.21730; PMID: 20872801
- Ma X, Ruan G, Wang Y, Li Q, Zhu P, Qin YZ, et al. Two single-nucleotide polymorphisms with linkage disequilibrium in the human programmed cell death 5 gene 5′ regulatory region affect promoter activity and the susceptibility of chronic myelogenous leukemia in Chinese population. Clin Cancer Res 2005; 11:8592 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-05-0039; PMID: 16361542
- Gu L, Jiang Y, Wang Y, Yao W, Sun D, Ka W, et al. TFAR19 gene changes the biophysical properties of murine erythroleukemia cells. Cell Biochem Biophys 2005; 43:355 - 63; http://dx.doi.org/10.1385/CBB:43:3:355; PMID: 16247177
- Spinola M, Meyer P, Kammerer S, Falvella FS, Boettger MB, Hoyal CR, et al. Association of the PDCD5 locus with lung cancer risk and prognosis in smokers. J Clin Oncol 2006; 24:1672 - 8; http://dx.doi.org/10.1200/JCO.2005.04.4339; PMID: 16549820
- Shi L, Song Q, Zhang Y, Lou Y, Wang Y, Tian L, et al. Potent antitumor activities of recombinant human PDCD5 protein in combination with chemotherapy drugs in K562 cells. Biochem Biophys Res Commun 2010; 396:224 - 30; http://dx.doi.org/10.1016/j.bbrc.2010.04.068; PMID: 20398626
- Li H, Wang Q, Gao F, Zhu F, Wang X, Zhou C, et al. Reduced expression of PDCD5 is associated with high-grade astrocytic gliomas. Oncol Rep 2008; 20:573 - 9; PMID: 18695908
- Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet 2002; 359:1011 - 8; http://dx.doi.org/10.1016/S0140-6736(02)08091-1; PMID: 11937180
- Yin A, Jiang Y, Zhang X, Zhao J, Luo H.. Transfection of PDCD5 sensitizes colorectal cancer cells to cisplatin-induced apoptosis in vitro and in vivo. Eur J Pharmacol 2010; 649:120 - 126
- Chen C, Zhou H, Xu L, Xu D, Wang Y, Zhang Y, et al. Recombinant human PDCD5 sensitizes chondrosarcomas to cisplatin chemotherapy in vitro and in vivo. Apoptosis 2010; 15:805 - 13; http://dx.doi.org/10.1007/s10495-010-0489-5; PMID: 20349137
- Ruan GR, Chen SS, Chang Y, Li JL, Qin YZ, Li LD, et al. [Adenovirus-mediated PDCD5 gene transfer sensitizes apoptosis of K562 cells induced by etoposide]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2007; 15:936 - 40; PMID: 17956665
- Ruan GR, Zhao HS, Chang Y, Li JL, Qin YZ, Liu YR, et al. Adenovirus-mediated PDCD5 gene transfer sensitizes K562 cells to apoptosis induced by idarubicin in vitro and in vivo. Apoptosis 2008; 13:641 - 8; http://dx.doi.org/10.1007/s10495-008-0206-9; PMID: 18401719
- Wang Y, Li D, Fan H, Tian L, Zhong Y, Zhang Y. Cellular uptake of exogenous human PDCD5 protein. J Biol Chem 2006; 281:24803 - 17; http://dx.doi.org/10.1074/jbc.M600183200; PMID: 16754680
- Wang D, Lippard SJ.. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov 2005; 4:307 - 320
- Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 2003; 22:7265 - 79; http://dx.doi.org/10.1038/sj.onc.1206933; PMID: 14576837
- Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006; 25:4798 - 811; http://dx.doi.org/10.1038/sj.onc.1209608; PMID: 16892092
- Korsmeyer SJ. BCL-2 gene family and the regulation of programmed cell death. Cancer Res 1999; 59:Suppl 1693s - 700s; PMID: 10197582