Abstract
Osteosarcoma (OS) is the most common human primary malignant bone tumor in children and young adults with poor prognosis because of their high metastatic potential. Identification of key factors that could regulate the aggressive biologic behavior of OS, particularly with respect to metastasis, would be necessary if significant improvements in therapeutic outcome are to occur. In this study, we carefully evaluated the potential role of IL-17A/IL-17RA interaction in metastasis of OS. We found that serum IL-17A was higher in OS patients with metastasis and was associated with their clinical stage. The elevated expression of IL-17RA was observed in tumor tissue from OS patients with metastasis. Of note, we showed that IL-17A could promote the metastasis of U-2 OS cells which expression high IL-17RA, but not MG63 cells which expression low IL-17RA. Further, we revealed that downregulation of IL-17RA in U-2 cells could abrogated the enhanced metastasis induced by IL-17A, while upregulation of IL-17RA in MG63 cells could elevate their response to IL-17A and exerted enhanced metastasis. We observed that IL-17A/IL-17RA interaction promoted the expression of VEGF, MMP9 and CXCR4 in OS cells, which might partly explain the enhanced metastasis of OS cells. Furthermore, we showed that Stat3 activity was crucial for IL-17A/IL-17RA interaction to promote OS metastasis. Finally, we confirmed that IL-17A/IL-17RA interaction promoted the metastasis of OS in nude mice. Our findings might provide a mechanistic explanation for metastasis of OS in vivo, and suggested that targeting IL-17A signaling was a novel promising strategy to treat patients with OS.
Keywords: :
Introduction
Osteosarcoma (OS) is the most common human primary malignant bone tumor in children and young adults, which account for approximately 60% of malignant bone tumors in the first two decades of life.Citation1,Citation2 OS is locally destructive and has a high metastatic potential.Citation2,Citation3 OS metastasis appear most frequently in the lung and are the main cause of death for patients with OS.Citation4,Citation5 Despite aggressive treatment including surgery and chemotherapy, little improvement in survival times was achieved in patients with OS over the past 15 years even with significant efforts directed at the incorporation of novel therapeutic approaches.Citation6-Citation8 Although the 5-year survival indeed increased to around 60%, the 5-year survival of OS patients with metastasis was still about 30%.Citation4,Citation9,Citation10 Thus, OS patients with metastasis presented further worse clinical results, and more effective treatments and/or a more personalized therapy are still needed.Citation4 Therefore, the identification of key factors that could regulate the aggressive biologic behavior of OS, particularly with respect to metastasis, would be necessary.
IL-17A is a proinflammatory cytokine which is mainly secreted by T cells, and has been shown to play important roles in inflammatory autoimmune disease.Citation11-Citation14 Accumulating data showed that the role of IL-17A in cancer initiation, growth, and metastasis was crucial but controversial.Citation15-Citation17 Forced overexpression of IL-17A ectopically in tumor cells could either suppress tumor progression through enhanced antitumor immunity in immune-competent mice or promote tumor progression through an increase in inflammatory angiogenesis in immune-deficient mice.Citation18-Citation21 In OS, previous study showed that U-2 OS cells which expressed higher level of IL-17RA were more sensitive to IL-17A, and secreted higher amounts of vascular endothelial growth factor (VEGF), whereas MG63 expressed lower level of IL-17RA, was not sensitive to IL-17A and secreted lower amount of VEGF, suggesting that IL-17RA expression was correlated with VEGF secretion.Citation22 Given that VEGF is well established as one of the key regulators of the new blood vessel formation (angiogenesis) which is a fundamental event in the process of tumor growth and metastatic dissemination, and was an important negative prognostic factor in OS,Citation23 we hypothesized that IL-17A/IL-17RA interaction might be an important factor involved in the OS metastasis.
In this study, we carefully evaluated the direct effect of IL-17A/IL-17RA interaction on the metastasis of OS. Our findings might enlarge our understanding of IL-17A/IL-17RA interaction in progression of OS, suggesting that targeting IL-17A/IL-17RA pathway was a novel promising strategy to treat patients with OS.
Results
Elevated level of serum IL-17A in OS patients with metastasis
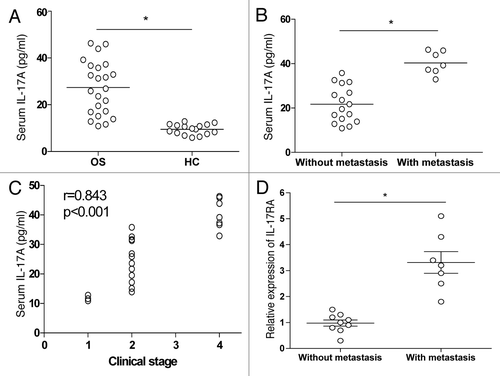
To evaluate the potential role of IL-17A in metastasis of OS, the OS patients and healthy controls were enrolled and assayed for their serological level of IL-17A in peripheral blood. As shown in , we found a higher expression of serum IL-17A in OS patients compared with the healthy controls (p < 0.05). To determine the possible effect of IL-17A on the metastasis of OS, we analyzed the expression of serum IL-17A in OS patients with or without metastasis, and found that the expression of serum IL-17A in patients with metastasis was significantly higher than that in patients without metastasis (, p < 0.05). To further evaluate the association of IL-17A expression with tumor biology, the relationship between serum IL-17A and the clinical pathological features were analyzed. We revealed that the high serum IL-17A expression was positively correlated with the clinical stages in OS patients (, p < 0.05). In contrast, high serum IL-17A expression did not correlate with the age, sex, and histological subtype (data not shown). To further evaluate the possible direct effect of IL-17A on the metastasis of OS cells, we analyzed the expression of IL-17RA mRNA in tumor tissues in 16 OS patients using Real-time PCR. As shown in , we found that IL-17RA expression was higher in tumor tissues from OS patients with metastasis than that in tumor tissues from OS patients without metastasis (p < 0.05). These findings suggested that IL-17A/IL-17RA signaling might be involved in metastasis of OS.
Figure 1. Elevated IL-17A expression in OS patients. (A) The serological level of IL-17A was determined by ELISA in 23 NSCLC patients and 16 healthy controls. (B) The serological level of IL-17A in 16 OS patients without metastasis and 7 patients with metastasis. (C) The correlation between serum IL-17A and clinical stage of 23 OS patients was analyzed. (D) The relative expression of IL-17RA mRNA was detected in tumor tissue from 9 OS patients without metastasis and 7 OS patients with metastasis respectively. Each dot represented the data from one OS patient. *p < 0.05.
IL-17A/IL-17RA interaction promoted the metastasis of OS cells in vitro
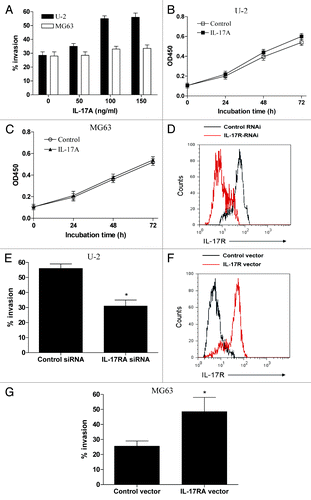
To further evaluate the direct effect of IL-17A on the metastasis of OS cells, OS cell line U-2 OS cells with high expression of IL-17RA and MG63 cells with low expression of IL-17RA were detected for their response to IL-17A stimulation. We did not found significant secretion of IL-17A from U-2 cells and MG63 cells (data not shown). However, we found that the invasive potential of U-2 cells was significantly enhanced by IL-17A treatment in a dose dependent manner (, p < 0.05). In contrast, we observed a relative weak effect of IL-17A on the invasion of MG63 cells (, p > 0.05). As noted, 100ng/ml of IL-17A was then used for the following experiments in vitro. Further, we determined the potential effect of IL-17A on the growth of OS cells, and found that IL-17A stimulation had no significant effect on the growth of U-2 cells and MG63 cells (, p > 0.05). To determine whether the IL-17A/IL-17RA interaction was responsible for the enhanced invasive potential of U-2 OS cells, these cells were transfected with siRNA against IL-17RA and then stimulated with IL-17A. We found that transfection of IL-17RA siRNA significantly decreased the expression of IL-17RA in U-2 cells (, p < 0.05). Of important, we showed that downregulation of IL-17RA in U-2 OS cells abrogated their enhanced invasion induced by IL-17A (, p < 0.05). To further confirm the effect of IL-17A/IL-17RA interaction on metastasis of OS cells, MG63 cells were transfected with IL-17RA expression vector and assayed for their response to IL-17A. We found that transfection of IL-17RA expression vector effectively elevated the IL-17RA expression in MG63 cells and enhanced the invasion of MG63 cells in response to IL-17A (, p < 0.05). These data suggested the IL-17A/IL-17RA interaction could promote the metastasis of OS cells in vitro.
Figure 2. IL-17A/IL-17RA interaction promoted the metastasis of OS cells in vitro. (A) 2 × 106 U-2 cells or MG63 cells were treated with the indicated dose IL-17A for 48 h and then assayed for their invasive potential. (B and C) U-2 OS cells (B) or MG63 cells (C) were incubated with 100 ng/ml of IL-17A for the indicated time and assayed for their growth by MTT assay. (D and E) U-2 cells were transiently transfected with IL-17RA siRNA or the control siRNA, and then stimulated with 100ng/ml of IL-17A for 48 h. (F and G) MG63 cells were transiently transfected with IL-17RA expression vector or control vector, and then stimulated with 100 ng/ml of IL-17A for 48 h. Each bar represents the means (± SD) in triplicate from three independent experiments. *p < 0.05.
IL-17A/IL-17RA interaction upregulated the expression of VEGF, MMP9 and CXCR4
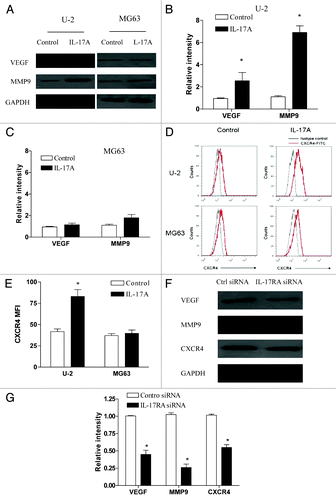
To further our understanding of the effect of IL-17A/IL-17RA interaction on OS metastasis, we analyzed the expression of VEGF, MMP9 and CXCR4, which are important molecules associated with tumor metastasis, in OS cells in response to IL-17A. As shown in −C, we found that the protein level of VEGF and MMP9 was significantly elevated by IL-17A treatment in U-2 cells but not in MG63 cells (p < 0.05). Besides, we revealed that the expression of CXCR4 on U-2 OS cells was also significantly upregulated by IL-17A treatment (, p < 0.05). In contrast, we found no significant changes of CXCR4 expression on MG63 cells after IL-17A treatment (, p > 0.05). Further, we showed that downregulation of IL-17RA using siRNA significantly abrogated the elevated expression of VEGF, MMP9 and CXCR4 induced by IL-17A in U-2 cells (, p < 0.05). In addition, we also found that IL-17RA overexpression in MG63 cells effectively enhanced the expression VEGF, MMP9 and CXCR4 in response to IL-17A (data not shown). These findings strongly demonstrated that IL-17A/IL-17RA interaction promoted the expression of VEGF, MMP9 and CXCR4 in OS cells, which might partly explain their effect on OS metastasis.
Figure 3. IL-17A/IL-17RA interaction elevated the expression of VEGF, MMP9 and CXCR4 in OS cells. (A−C) U-2 OS cells or MG63 cells were incubated with IL-17A (100 ng/ml) for 48 h and then assayed for their expression level of VEGF and MMP9 using western blot. Each bar represents the means (± SD) of the relative intensity of VEGF and MMP9 calculated from three independent experiments. (D and E) U-2 OS cells or MG63 cells were incubated with IL-17A (100 ng/ml) for 48 h and then assayed for their expression of CXCR4 using FACS analysis respectively. The MFI (mean fluorescence intensity) in each group was calculated (E). (F and G) U-2 cells transiently transfected with IL-17RA siRNA (100 nM) or the control siRNA (100 nM) were stimulated with 100ng/ml of IL-17A for 48 h and then assayed for their expression of VEGF, MMP9 and CXCR4. Each bar represents the means (± SD) of their relative intensity calculated from three independent experiments. *p < 0.05.
Stat3 activity was crucial for IL-17A/IL-17RA interaction to promote OS metastasis
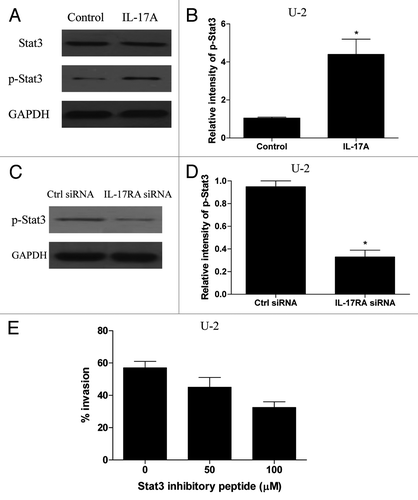
Given that IL-17A/IL-17RA interaction could promote the metastasis of OS cells, we next sought to elucidate the possible mechanisms. It is well acknowledged that Stat3 activation in tumor cells and tumor-associated inflammatory cells played a critical role in tumor progression by augmenting tumor survival and tumor angiogenesis, and suppressing antitumor immunity.Citation24-Citation26 Recent study showed that IL-17A could stimulate Stat3 activation in B16 melanoma and MB49 bladder carcinoma cells.Citation27 We therefore determined whether the IL-17A/IL-17RA interaction could promote the Stat3 activity in OS cells. As shown in , we found that the expression of phospholated Stat3 increased obviously in U-2 OS cells after IL-17A treatment (p < 0.05). Further, we showed that transfection with IL-17RA siRNA significantly inhibited the expression of phospholated Stat3 induced by IL-17A in U-2 cells (, p < 0.05). In contrast, we found no significant changes of phospholated Stat3 expression in MG63 cells after IL-17A stimulation (data not shown). These results suggested that IL-17A/IL-17RA interaction effectively promoted the Stat3 activity in OS cells. To further elucidate the potential role of Stat3 activity during the enhanced invasion induced by IL-17A/IL-17RA interaction in OS cells, U-2 cells were pretreated with Stat3-specific inhibitory peptide and then incubated with IL-17A. As shown in , we found that administration of Stat3 inhibitory peptide effectively impaired the enhanced invasive potential of U-2 cells induced by IL-17A in a dose dependent manner (p < 0.05). These data suggested that Stat3 activity was crucial for IL-17A/IL-17RA interaction to promote the metastasis of OS cells.
Figure 4. IL-17A/IL-17RA interaction induced Stat3 activity in OS cells. (A and B) U-2 OS cells were incubated with IL-17A (100 ng/ml) for 48 h and then assayed for their expression of total Stat3 and phospho-Stat3 using western blot. Each bar represents the means (± SD) of the relative intensity calculated from three independent experiments. (C and D) U-2 cells transiently transfected with IL-17RA siRNA (100 nM) or the control siRNA (100 nM) were stimulated with 100 ng/ml of IL-17A for 48 h and then assayed for their expression of phospho-Stat3 using western blot. Each bar represents the means (± SD) of their relative intensity calculated from three independent experiments. (E) U-2 cells were cultured with IL-7A (100 ng/ml), and the indicated dose of Stat3-specific inhibitory peptide was added to the cultures 1 h before stimulation. After 48 h, the U-2 cells were analyzed for their invasive potential. Each bar represents the means (± SD) in triplicate calculated from three independent experiments. *p < 0.05.
IL-17A/IL-17RA interaction enhanced the metastasis of OS cells in vivo
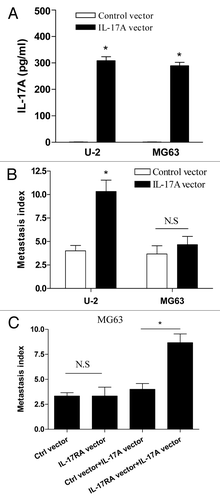
Finally, to confirm the potential effect of IL-17A/IL-17RA interaction on metastasis of OS cells in vivo, groups of nude mice were challenged with U-2 cells which were stably transfected with human IL-17A expression vector. As shown in , we found that the expression level of IL-17A increased significantly in U-2 cells transfected with IL-17A expression vector. Importantly, we found that enforced expression of IL-17A dramatically enhanced the metastasis index of U-2 OS cells in nude mice (, p < 0.05). Consistent with our above finding, we found no significant effect of enforced expression of IL-17A on the metastasis of MG63 cells in vivo (, p > 0.05). To further confirm the effect of IL-17RA expression on their distinct response to IL-17A, nude mice were challenged with MG63 cells which were stably co-transfected with IL-17A and IL-17RA expression vector. We found that co-transfection with IL-17A and IL-17RA expression vector effectively enhanced the metastasis of MG63 cells in vivo (, p < 0.05). Combing these data demonstrated that IL-17A/IL-17RA interaction promoted the metastasis of OS cells in vivo.
Figure 5. IL-17A/IL-17RA interaction enhanced the metastasis of OS cells in vivo. (A) 1 × 106 U-2 cells or MG63 cells stably transfected with IL-17A expression vector or control vector were incubated at 6-well plates respectively. After 48 h, the concentration of supernatant IL-17A was determined by ELISA assay. (B) Groups of eight nude mice were challenged with 2 × 106 of U-2 cells or MG63 cells which were stably transfected with IL-17A expression vector or control vector, respectively. (C) Groups of eight nude mice were challenged with 2 × 106 G63 cells which were stably co-transfected with IL-17A expression vector and IL-17RA expression vector, or the corresponding control vector, respectively. After 30 d, the metastatic index to lung was determined. Each bar represents the means (± SD) in each group. *p < 0.05
Discussion
The link between inflammation and carcinogenesis is well known, experiments have implicated many components of the inflammatory cascade such as prostaglandin E2 and IL-6 as key players in tumor development, growth, and metastasis.Citation28-Citation30 Recently, numerous immune regulatory functions have been reported for the IL-17A family of cytokines, and most notably, IL-17A was involved in inducing and mediating inflammatory responses.Citation11-Citation13 In contrast, the role of IL-17A in cancer initiation, growth, and metastasis was very controversial.Citation15-Citation17 In present study, we demonstrated that IL-17A/IL-17RA interaction could promote the growth and metastasis of OS cells in vivo. Our findings might enlarge our understanding of the crucial role of IL-17A in tumor immunity, and the association between inflammatory conditions and cancer.
Accumulating evidence showed that IL-17A-positive cells were frequently present in multiple inflammation-associated cancers and that IL-17A promoted angiogenesis in tumor models.Citation31,Citation32 In this study, we first showed that the level of serum IL-17A in OS patients was higher than that in control health donors. Further, the high level of serum IL-17A was associated with the clinical stage of OS patients. The expression of IL-17RA in tumor tissues was higher in OS patients with metastasis than that in OS patients without metastasis. These findings indicated that IL-17A/IL-17RA interaction might be involved in the metastasis of OS. Further, we showed that IL-17A could significantly promote the metastasis of U-2 OS cells which expressed high level of IL-17RA, but not MG63 cells which expressed low level of IL-17RA. Of note, we found that downregulation of intrinsic IL-17RA expression could abrogate the effect of IL-17A on the metastasis of U-2 cells. We also demonstrated that overexpression of IL-17RA in MG63 cells could elevate their response to IL-17A and exerted the enhanced metastasis in vitro. Thus, our data extended previous studies by providing the direct evidence to elucidate the effect of IL-17A/IL-17RA interaction on the metastasis of OS.
Accumulating data showed that soluble factors, surface molecules on tumor cells are involved in tumor evasion from immune surveillance.Citation33 Here we found that the protein expression of VEGF and CXCR4, which are closely correlated with metastasis of tumors,Citation23,Citation33 were upregulated in IL-17A treated U-2 cells. Furthermore, we found that IL-17A could enhance the protein expression of MMP-9, which is required for the proteolytic modifications of basement membranes and extracellular matrices during angiogenesis,Citation33 in U-2 cells. Therefore, these results might partly explain the enhanced metastasis of IL-17A treated U-2 cells. Our data were in line with the previous study which showed that IL-17RA expression correlated with VEGF secretion and IL-17A sensitivity and thus represented a marker of tumor metastasis potential.Citation22
IL-17A had recently been reported to stimulate Stat3 activation through a positive feedback loop in inflammatory cells, fibroblasts, as well as the growth of B16 melanoma and MB49 bladder carcinoma.Citation27 As Stat3 activation in tumor cells and tumor-associated inflammatory cells played a critical role in tumor progression by augmenting tumor survival and tumor angiogenesis, and suppressing antitumor immunity,Citation24-Citation26 we therefore detected the Stat3 activity in OS cells in response to IL-17A/IL-17RA interaction. Indeed, here we found that IL-17A/IL-17RA interaction could effectively induce the Stat3 activation in OS cells. Further, we showed that Stat3 inhibitory peptide significantly abrogated the enhanced metastasis of OS cells induced by IL-17A/IL-17RA interaction. However, the precise mechanisms underlie the direct effect of IL-17A/IL-17RA interaction on the tumor biology of OS cells undoubtedly needed successive studies.
In summary, here we demonstrated that IL-17A/IL-17RA interaction could promote the metastasis of OS. Our findings might provide a mechanistic explanation for the metastasis of OS in vivo, and suggested that targeting IL-17A/IL-17RA pathway was a novel promising strategy to treat patients with OS.
Materials and Methods
Patients
A total of 23 new diagnosed osteosarcoma patients were recruited for the study with a median age of 16 y. The male/female ratio was 17:6 and 16/23 patients were non-metastatic. Patients’ distribution according to AJCC staging system showed stage I in 3, stage II in 13, and stage IV in 7 patients. This study was approved by the local ethics committee and written informed consent was obtained from each patient. None of the patients received any anticancer therapy prior to sample collection. Sixteen healthy controls at the corresponding age to OS patients were enrolled.
Real-time PCR
Total cellular RNA was prepared by the guanidinium thiocyanate-acid phenol method. Residual DNA was eliminated with DNase I. Reverse transcription was performed as described by the manufacturer. Genomic DNA was used as a template. IL-17RA levels were measured by SYBR Green-based Real-time PCR using Light Cycler (Roche). The PCR contained 0.3 mM of each primer, 3 mM MgCL2 and 0.75 U of Platinum Taq polymerase (Invitrogen). The primer sequences were designed to bracket an intron to avoid amplification of genomic DNA. Their sequences were as follows; IL-17RA primers: 5′-ACCCAAACCACCAGTCC-3′ and 5′-GCCCGTGATGAACCAGTA-3′. PCR cycling conditions consisted of 95°C for 6 min, followed by 45 cycles of 95°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec. Cycle threshold (CT) values were compared against a standard curve to estimate starting amounts of mRNA, and the relative expression of IL-17RA mRNA between samples was estimated by normalizing these values against 18S rRNA CT values were generated using a preoptimized 18S rRNA primer set (Applied Biosystems).
Mice
Female BALB/c nude mice of 6 weeks old were obtained and housed in a pathogen-free mouse colony at the Center of Experimental Animals of our institution. All animal experiments were performed according to the Guide for the Care and Use of Medical Laboratory Animals and with the ethical approval of the Shanghai Medical Laboratory Animal Care and Use Committee.
Reagents and cell line
The recombinant human IL-17A and human IL-17A quantikine ELISA kit were all purchased from R&D Systems. The siRNA against human IL-17RA was used as previously described.Citation34 Cell-permeable STAT3 inhibitory peptide was purchased from Calbiochem. The U-2 OS and MG63 cells were obtained from ATCC and maintained at 37°C under 5% CO2 in complete RPMI 1640 medium (GIBCO) containing 10% heat-inactivated fetal bovine serum supplemented with 2 mM glutamine, 100 IU/ml penicillin and 100 mg/ml streptomycin sulfate.
MTT assay
Cells were seeded at 3 × 103 cells each well and incubated in the presence or absence of recombinant IL-17A in 96-well plates for 72 h. Assessment of cell proliferation was measured using commercially MTT cell proliferation kit (Cayman) according to the manufacturer’s instructions.
Invasive assay
The BD Biocoat Matrigel Invasion Chamber assay was performed as described by the manufacturers (8 μm, BD Bioscience). Briefly, the Matrigel inserts were rehydrated and 5 × 104 testing cells were re-suspended in 0.5 mL of serum-free media and then seeded onto the upper chamber of Matrigel-coated filters. In the lower chambers, 0.75 mL of complete medium was added as a chemoattractant. The whole chamber was placed in one well of a 24-well plate, and cells were cultured in routine conditions. After 24 h, the cells on the upper side of the chamber were scraped, and the ones on the lower side of the chamber were fixed by methanol, stained with hematoxylin, and invaded cells were counted under the microscope. Five predetermined fields were counted for each membrane, and the mean values from three independent experiments in triplicates were used. Data are expressed as the percentage of invasion through the Matrigel Matrix and membrane relative to the migration through the control membrane according to the manufacturer’s manual.
Plasmid construction and transfection
Human IL-17A and IL-17RA expression vector were generated as previously described.Citation35 U-2 cells or MG63 cells were transfected with the human IL-17A expression vector and/or IL-17RA expression vector using LipofectAMINE (Invitrogen Life Technologies) and selected in RPMI 1640 with 10% FCS, 2 mM L-glutamine, 1 mM sodium pyruvate, 0.1mM NEAA, 100IU/ml penicillin, 100 μg/ml streptomycin, and 1000 μg/ml G418. As a control, a vector carrying only neomycin phosphotransferase gene (Neo) was used.
Flow cytometry
Flow cytometry was performed on a FACS Calibur (BD Biosciences) with CellQuest Pro software using directly conjugated mAbs against the following markers: IL-17RA-FITC or CXCR4-PE with corresponding isotype-matched controls (either BD Biosciences or eBioscience Systems). In brief, cells (1 × 106 cells) were first incubated with fixation buffers for 45 min. After washing twice, cells were incubated with the specific mAb for 30 min at 4°C in 0.1% bovine serum albumin/PBS. Following extensive washing, the cells were analyzed.
Western blotting
Cells were lysed with M-PER Protein Extraction Reagent (Pierce) supplemented with a protease inhibitor cocktail. Cytoplasmic and nuclear extracts were prepared using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce). After centrifugation at 13,000 g under 4°C for 15 min, the supernatants were collected, and the protein concentration of the extracts was measured by BCA Protein Assay (Pierce) according to manufacturer’s instructions. Twenty micrograms of the protein were loaded onto 10% SDS-polyacrylamide gels and transferred for 90 min at 100 V onto polyvinylidene fluoride membranes using a wet transfer system. The membranes were washed in 5% skim milk in phosphate buffered saline plus 0.05% Tween 20 (PBST) for 2h in order to block nonspecific protein binding sites on the membrane. Immunoblotting was performed using monoclonal antibodies to phospho-Stat3, Stat3, VEGF, CXCR4 and MMP9 (Sigma and Cell Signaling Technology) at a dilution of 1:1000 in nonfat milk Tris buffer. The membrane was then washed in PBST, probed with a secondary anti-rabbit antibody conjugated to horseradish peroxidase at a dilution of 1:5000, developed using an ECL Western Blotting KIT (Pierce), and exposed to X-ray film (Kodak).
Metastasis index to lung in vivo
Groups of nude mice were challenged with tumor cells as described previously.Citation36 Metastasis index to lung was calculated as previously described.Citation37 Briefly, 30 d after challenge, tumor bearing mice were killed and the lungs were evaluated for number and size of metastasis. Tumor metastasis could be clearly recognized as raised, hemorrhagic, opaque, and dense nodules, distinguishable from the smooth, glistening, spongy, and pink normal lung parenchyma. The metastatic foci were confirmed microscopically in doubtful cases. To obtain an approximate assessment of the total metastatic tumor burden in the lungs using “metastatic index,” each metastasis less than 0.5 mm in diameter was graded as 1, between 0.5 and 1 mm as Grade 2, between 1.0 and 2 mm as Grade 3, and > 2 mm as Grade 4. All the grade scores were then added to determine the metastatic index for a given animal, and the mean index was then calculated for a given control or experimental group of animals.
Statistical analyses
Statistical analyses of the data were performed with the aid of analysis programs in SPSS12.0 software. Statistical evaluation was performed using two-way analysis of variance (ANOVA; p < 0.05) using the program PRISM 4.0 (GraphPad Software Inc., San Diego, CA, USA).
| Abbreviations: | ||
| IL-17A | = | Interlukin-17A |
| IL-17RA | = | Interlukin-17 receptor A |
| OS | = | osteosarcoma |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by Qingdao Public Sphere Support Program (2010KZJ-9), Fund of Science and Technology Department of Pudong New Area (PKJ2011-Y33), National Natural Science Foundation of China (81071744), International Cooperation Foundation of Guizhou Province (09C399), Shanghai Pudong New Area Academic Leader in Health System (PWRd2010–01), Specific Foundation for the Scientific Educational Talent of President of Guizhou Province (09C457, 10–49), Project of Guizhou provincial Department of Science and Technology (2009C491), Zunyi Medical College Start-up Fund (2008F-329) and Basic Research Program supported by the Shanghai Committee of Science and Technology (11JC1410900).
References
- Ma O, Cai WW, Zender L, Dayaram T, Shen J, Herron AJ, et al. MMP13, Birc2 (cIAP1), and Birc3 (cIAP2), amplified on chromosome 9, collaborate with p53 deficiency in mouse osteosarcoma progression. Cancer Res 2009; 69:2559 - 67; http://dx.doi.org/10.1158/0008-5472.CAN-08-2929; PMID: 19276372
- Yan K, Gao J, Yang T, Ma Q, Qiu X, Fan Q, et al. MicroRNA-34a inhibits the proliferation and metastasis of osteosarcoma cells both in vitro and in vivo. PLoS One 2012; 7:e33778; http://dx.doi.org/10.1371/journal.pone.0033778; PMID: 22457788
- Walkley CR, Qudsi R, Sankaran VG, Perry JA, Gostissa M, Roth SI, et al. Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Genes Dev 2008; 22:1662 - 76; http://dx.doi.org/10.1101/gad.1656808; PMID: 18559481
- Ando K, Mori K, Verrecchia F, Marc B, Rédini F, Heymann D. Molecular alterations associated with osteosarcoma development. Sarcoma 2012; 2012:523432; http://dx.doi.org/10.1155/2012/523432; PMID: 22448123
- Bacci G, Ferrari S, Longhi A, Perin S, Forni C, Fabbri N, et al. Pattern of relapse in patients with osteosarcoma of the extremities treated with neoadjuvant chemotherapy. Eur J Cancer 2001; 37:32 - 8; http://dx.doi.org/10.1016/S0959-8049(00)00361-0; PMID: 11165127
- Paoloni M, Davis S, Lana S, Withrow S, Sangiorgi L, Picci P, et al. Canine tumor cross-species genomics uncovers targets linked to osteosarcoma progression. BMC Genomics 2009; 10:625; http://dx.doi.org/10.1186/1471-2164-10-625; PMID: 20028558
- Clark JC, Dass CR, Choong PF. A review of clinical and molecular prognostic factors in osteosarcoma. J Cancer Res Clin Oncol 2008; 134:281 - 97; http://dx.doi.org/10.1007/s00432-007-0330-x; PMID: 17965883
- Fossey SL, Bear MD, Kisseberth WC, Pennell M, London CA. Oncostatin M promotes STAT3 activation, VEGF production, and invasion in osteosarcoma cell lines. BMC Cancer 2011; 11:125; http://dx.doi.org/10.1186/1471-2407-11-125; PMID: 21481226
- Bacci G, Longhi A, Cesari M, Versari M, Bertoni F. Influence of local recurrence on survival in patients with extremity osteosarcoma treated with neoadjuvant chemotherapy: the experience of a single institution with 44 patients. Cancer 2006; 106:2701 - 6; http://dx.doi.org/10.1002/cncr.21937; PMID: 16691623
- Longhi A, Errani C, De Paolis M, Mercuri M, Bacci G. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev 2006; 32:423 - 36; http://dx.doi.org/10.1016/j.ctrv.2006.05.005; PMID: 16860938
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 2007; 25:821 - 52; http://dx.doi.org/10.1146/annurev.immunol.25.022106.141557; PMID: 17201677
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol 2009; 27:485 - 517; http://dx.doi.org/10.1146/annurev.immunol.021908.132710; PMID: 19132915
- Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity 2004; 21:467 - 76; http://dx.doi.org/10.1016/j.immuni.2004.08.018; PMID: 15485625
- Bettelli E, Oukka M, Kuchroo VKT. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol 2007; 8:345 - 50; http://dx.doi.org/10.1038/ni0407-345; PMID: 17375096
- Martin-Orozco N, Dong C. The IL-17/IL-23 axis of inflammation in cancer: friend or foe?. Curr Opin Investig Drugs 2009; 10:543 - 9; PMID: 19513943
- Ngiow SF, Smyth MJ, Teng MW. Does IL-17 suppress tumor growth?. Blood 2010; 115:2554 - 5, author reply 2556-7; http://dx.doi.org/10.1182/blood-2009-11-254607; PMID: 20339108
- Ji Y, Zhang W. Th17 cells: positive or negative role in tumor?. Cancer Immunol Immunother 2010; 59:979 - 87; http://dx.doi.org/10.1007/s00262-010-0849-6; PMID: 20352428
- Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautès-Fridman C, et al. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood 2002; 99:2114 - 21; http://dx.doi.org/10.1182/blood.V99.6.2114; PMID: 11877287
- Hirahara N, Nio Y, Sasaki S, Minari Y, Takamura M, Iguchi C, et al. Inoculation of human interleukin-17 gene-transfected Meth-A fibrosarcoma cells induces T cell-dependent tumor-specific immunity in mice. Oncology 2001; 61:79 - 89; http://dx.doi.org/10.1159/000055357; PMID: 11474253
- Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood 2003; 101:2620 - 7; http://dx.doi.org/10.1182/blood-2002-05-1461; PMID: 12411307
- Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood 2009; 114:357 - 9; http://dx.doi.org/10.1182/blood-2008-09-177360; PMID: 19289853
- Honorati MC, Cattini L, Facchini A. Possible prognostic role of IL-17R in osteosarcoma. J Cancer Res Clin Oncol 2007; 133:1017 - 21; http://dx.doi.org/10.1007/s00432-007-0296-8; PMID: 17690908
- Bajpai J, Sharma M, Sreenivas V, Kumar R, Gamnagatti S, Khan SA, et al. VEGF expression as a prognostic marker in osteosarcoma. Pediatr Blood Cancer 2009; 53:1035 - 9; http://dx.doi.org/10.1002/pbc.22178; PMID: 19621435
- Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 2007; 7:41 - 51; http://dx.doi.org/10.1038/nri1995; PMID: 17186030
- Zhang X, Yue P, Page BD, Li T, Zhao W, Namanja AT, et al. Orally bioavailable small-molecule inhibitor of transcription factor Stat3 regresses human breast and lung cancer xenografts. Proc Natl Acad Sci U S A 2012; 109:9623 - 8; http://dx.doi.org/10.1073/pnas.1121606109; PMID: 22623533
- Lavecchia A, Di Giovanni C, Novellino E. STAT-3 inhibitors: state of the art and new horizons for cancer treatment. Curr Med Chem 2011; 18:2359 - 75; http://dx.doi.org/10.2174/092986711795843218; PMID: 21568920
- Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med 2009; 206:1457 - 64; http://dx.doi.org/10.1084/jem.20090207; PMID: 19564351
- Mantovani A. Role of inflammatory cells and mediators in tumor invasion and metastasis. Cancer Metastasis Rev 2010; 29:241; http://dx.doi.org/10.1007/s10555-010-9228-1; PMID: 20397039
- Solinas G, Marchesi F, Garlanda C, Mantovani A, Allavena P. Inflammation-mediated promotion of invasion and metastasis. Cancer Metastasis Rev 2010; 29:243 - 8; http://dx.doi.org/10.1007/s10555-010-9227-2; PMID: 20414701
- Danese S, Mantovani A. Inflammatory bowel disease and intestinal cancer: a paradigm of the Yin-Yang interplay between inflammation and cancer. Oncogene 2010; 29:3313 - 23; http://dx.doi.org/10.1038/onc.2010.109; PMID: 20400974
- Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol 2009; 183:4169 - 75; http://dx.doi.org/10.4049/jimmunol.0901017; PMID: 19767566
- Chen X, Xie Q, Cheng X, Diao X, Cheng Y, Liu J, et al. Role of interleukin-17 in lymphangiogenesis in non-small-cell lung cancer: Enhanced production of vascular endothelial growth factor C in non-small-cell lung carcinoma cells. Cancer Sci 2010; 101:2384 - 90; http://dx.doi.org/10.1111/j.1349-7006.2010.01684.x; PMID: 20825419
- Ren T, Wen ZK, Liu ZM, Liang YJ, Guo ZL, Xu L. Functional expression of TLR9 is associated to the metastatic potential of human lung cancer cell: functional active role of TLR9 on tumor metastasis. Cancer Biol Ther 2007; 6:1704 - 9; http://dx.doi.org/10.4161/cbt.6.11.4826; PMID: 17986857
- Wright JF, Bennett F, Li B, Brooks J, Luxenberg DP, Whitters MJ, et al. The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J Immunol 2008; 181:2799 - 805; PMID: 18684971
- Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, McAllister F, et al. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol 2005; 175:6177 - 89; PMID: 16237115
- Ren T, Xu L, Jiao S, Wang Y, Cai Y, Liang Y, et al. TLR9 signaling promotes tumor progression of human lung cancer cell in vivo. Pathol Oncol Res 2009; 15:623 - 30; http://dx.doi.org/10.1007/s12253-009-9162-0; PMID: 19319670
- Deodhar SD, James K, Chiang T, Edinger M, Barna BP. Inhibition of lung metastases in mice bearing a malignant fibrosarcoma by treatment with liposomes containing human C-reactive protein. Cancer Res 1982; 42:5084 - 8; PMID: 7139613