Abstract
NPRL2 is a tumor suppressor gene involved in the progression of human cancer. The present study investigated whether NPRL2 expression correlates with colorectal cancer (CRC) progression.
Colorectal tissue and peripheral blood samples were obtained from 62 patients with CRC, 38 patients with colorectal adenomas and 51 normal controls. NPRL2 mRNA levels in tissue samples and blood were measured using quantitative real-time PCR. NPRL2 protein expression was determined by immunohistochemistry.
NPRL2 protein expression in CRCs was significantly lower than in the adenomas or normal colorectal tissue. NPRL2 mRNA expression was significantly decreased in adenomas compared with normal controls (P < 0.0001) and it was further decreased in colorectal tumors compared with adenomas (P < 0.0001). NPRL2 mRNA levels expression correlated with tumor stage. In addition, NPRL2 mRNA levels in the blood correlated with the levels detected in tumors. Furthermore, receiver operating characteristic (ROC) analysis showed that NPRL2 expression in blood could distinguish colorectal adenomas and CRCs from normal controls.
NPRL2 mRNA expression in CRC tumor tissues and peripheral blood correlated with colorectal tumor progression. Based on our findings, we can conclude that NPRL2 mRNA blood levels could be a potentially useful marker for the detection of early stage adenomas and CRCs.
Introduction
Colorectal cancer (CRC) is a disease with very high morbidity and mortality rate worldwide. It ranks second as the cause of cancer related death in western countriesCitation1,Citation2 and its prevalence is increasing in developing countries such as Korea and China.Citation3,Citation4 CRC is potentially one of the most preventable diseases of all malignancies.Citation5-Citation8 Detection and removal of colorectal cancer at an early stage, or at adenoma stage, is commonly recognized as the most efficient approach to decrease CRC-related mortality.Citation4,Citation9 Therefore, efforts have been directed toward identifying non-invasive or minimally invasive tumor markers for the detection of adenoma and CRC at its early stages.
Although a variety of biological markers have been investigated, none of them is considered sufficient for the detection of early CRC or even adenoma patients.Citation10,Citation11 Therefore it is important to find a sensitive biomarker that can reliably report on the presence and progression of adenoma and CRC.
Colorectal carcinogenesis from adenoma to invasive carcinoma is a multi-step process in which activation of many oncogenes and inactivation of tumor suppressor genes has been described. Genetic changes are considered to be among the most important factors in the occurrence of cancer.Citation12 Consequently genetic alterations involved in the development of CRC have been proposed as potential markers of disease progression.Citation6,Citation13,Citation14
There have been many tumor suppressor genes reported and one of the recently described tumor suppressor candidates is nitrogen permease regulator-like 2 (NPRL2). The NPRL2 gene has been identified in a 120-kb homozygous deletion region located in chromosome 3p21.3 NPRL2 is expressed in many normal tissues including those of the heart, liver, skeletal muscle, kidney, and pancreas.Citation15,Citation16 Allelic loss of chromosomal region 3p21.3 has been found to be a frequent and early event in the development of several cancers. Recently intensive tumor suppressor gene (TSG) searches have been performed in the 3p21.3 region to identify one or more genes that could function as “gatekeepers” in the molecular pathogenesis of human cancers such as lung cancer, esophageal squamous cell carcinomas and hepatocellular carcinoma.Citation15,Citation17-Citation20 The NPRL2 gene (AF040707) is one of nine potential TSGs that were found in the 3p21.3 region.Citation16,Citation21
To date, the expression of NPRL2 mRNA in peripheral blood, as well as in colorectal adenoma and CRC has not yet been examined. Therefore, the aim of this study was to investigate the NPRL2 expression in colorectal adenomas and CRC at different stages of progression as well as in the corresponding blood samples in order to evaluate its potential use as a biomarker in CRC development and progression.
Results
Immunohistochemical analysis of NPRL2 expression in normal colorectal tissue, adenomas, and CRCs
We found that NPRL2 was expressed at various levels in normal colorectal tissues as well as in adenomas and CRCs. All 51 normal control samples showed high NPRL2 expression (NPRL2+++). In the adenoma group, only 13.2% cases (5 of 38) showed high NPRL2 expression (NPRL2+++) while 86.8% cases (33 of 38) showed lower NPRL2 expression (NPRL2++) compared with normal control samples (P < 0.0001). These results showed that the reduction of NPRL2 protein expression starts in the adenoma stage. Furthermore, NPRL2 protein expression in CRCs was significantly lower than in the adenomas. In addition, the cytoplasmic NPRL2 protein expression was detected in the majority of cells of stage I tumors, whereas only a small number of cells expressed a low level of NPRL2 protein in stage IV tumors (). In the stage CRC I–II group 18.1% (6 of 33) of samples with NPRL2++, 45.5% (15 of 33) of samples with NPRL2+, and 36.4% (12 of 33) of samples with NPRL2− were observed (adenoma group vs. stage CRC I–II group, P < 0.0001). While in the stage CRC III–IV group, 3.4% (1 of 29) of samples with NPRL2++, 24.1% (7 of 29) of samples with NPRL2+, and 72.5% (21 of 29) of samples with NPRL2− were detected (stage CRC I–II group vs. stage CRC III–IV group, P = 0.013) ().
Figure 1.NPRL2 protein expression in normal colorectal tissue, adenoma and colorectal carcinoma of different histological grades (immunohistochemical staining × 400). Normal: +++, with 98% positive strongly staining; adenoma: ++, with 90% positive moderately staining; CRC I: ++, with 75% positive moderately staining; CRC II: +, with 45% positive moderately staining; CRC III: +, with 78% positive weakly staining; CRC IV: −, with 10% positive weakly staining.
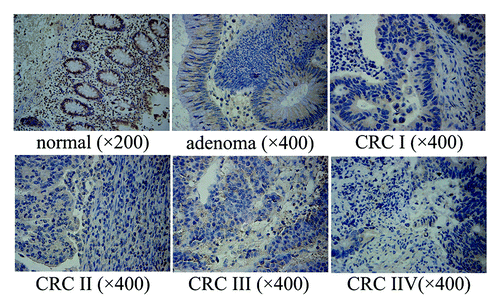
Table 1. Clinicopathological characteristics and NPRL2 expression of patients included in the study
CRC patients were also classified by histological grade. The data on histological grades was available on CRC tumors from 62 patients: 15 patients with well-differentiated CRC (grade 1), 38 patients with moderately differentiated CRC (grade 2), and 9 patients with poorly differentiated CRC (grade 3) were included in this study. In our study, well and moderately differentiated CRC tumors showed significantly higher NPRL2 expression than that observed in poorly differentiated tumors. In a well-differentiated group of tumors, there were 33.3% (5 of 15) of samples with NPRL2++, 60% (9 of 15) of samples with NPRL2+, and 6.7% (1 of 15) of samples with NPRL2−. In a group of moderately differentiated tumors, there were 5.2% (2 of 38) of samples with NPRL2++, 55.3% (21 of 38) of samples with NPRL2+ and 39.5% (15 of 38) of samples with NPRL2− (grade 1 vs. grade 2, P = 0.002, Fisher exact test). Finally, in the group of poorly differentiated tumors there were 11.1% (1 of 9) of samples with NPRL2+ and 88.9% (8 of 9) of samples with NPRL2− (grade 2 vs. grade 3, P = 0.0001, continuity correction χ2 test) (; ).
NPRL2 mRNA expression in normal colorectal tissue, adenomas, and CRCs
The NPRL2 mRNA expression was analyzed in a total of 62 CRCs, 38 colorectal adenomas, and 51 samples of normal colorectal tissue. The results of this analysis showed that NPRL2 expression was significantly decreased in adenomas compared with normal tissue (adenoma vs. normal, 0.92 ± 0.09 vs. 0.99 ± 0.08; P < 0.0001) and that NPRL2 mRNA expression was further significantly decreased in CRC I patients compared with adenomas (CRC I vs. adenoma, 0.92 ± 0.09 vs. 0.85 ± 0.10; P < 0.0001). In addition, NPRL2 expression decreased with tumor stage and disease progression (CRC I vs. CRC II, 0.85 ± 0.10 vs. 0.64 ± 0.09; P < 0.0001; CRC II vs. CRC III, 0.64 ± 0.09 vs. 0.500 ± 0.061; P < 0.0001; CRC III vs. CRC IV, 0.500 ± 0.061 vs. 0.352 ± 0.053, P < 0.0001) (; ).
Figure 2. (A) NPRL2 mRNA expression in colorectal tumors (stage I–IV) relative to normal controls. (*P < 0.0001). (B) NPRL2 mRNA levels in blood of CRC patients (tumor stage I–IV) relative to normal controls. (*P < 0.0001). (C) Correlation of tumor and blood NPRL2 mRNA expression in adenomas, colorectal tumors, and normal controls (r = 0.878; *P < 0.0001; R2 = 0.771).
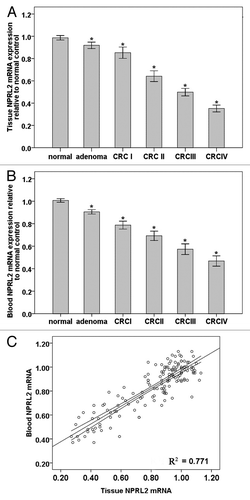
Table 2. Correlation between tissue NPRL2 expression detected by immunohistochemistry and clinicopathological variables in patients
NPRL2 mRNA levels in peripheral blood of CRC patients and its correlation with NPRL2 mRNA expression in tumors
Peripheral blood samples were available from 62 CRC patients (18 stage I, 15 stage II, 15 stage III, and 14 stage IV), 38 adenoma patients, and 51 age-matched healthy donors (control group). The results of our analysis showed that normal control blood samples were positive for NPRL2 mRNA. NPRL2 mRNA expression in the adenoma group was decreased compared with the NPRL2 mRNA levels in the normal control group (adenoma vs. normal, 0.91 ± 0.06 vs. 1.00 ± 0.06; P < 0.0001), and was further decreased in the CRC group compared with the adenoma group (CRC I vs. adenoma, 0.79 ± 0.07 vs. 0.91 ± 0.06, P < 0.0001; CRC II vs. CRC I, 0.69 ± 0.07 vs. 0.79 ± 0.07, P < 0.0001; CRC III vs. CRC II, 0.57 ± 0.09 vs. 0.69 ± 0.07, P < 0.0001; CRC IV vs. CRC III, 0.50 ± 0.08 vs. 0.57 ± 0.09, P < 0.0001) (; ). In addition blood NPRL2 mRNA levels were positively correlated with the NPRL2 mRNA expression in tumor tissue (r = 0.878, P < 0.0001) ().
Evaluation of blood NPRL2 mRNA level as a potential diagnostic marker for colorectal adenoma and CRC
Receiver operating characteristic (ROC) analysis was used to determine the potential use of blood NPRL2 mRNA levels in distinguishing colorectal adenomas and CRCs from normal controls ().
Figure 3. (A) Receiver-operating characteristics curve for healthy and adenoma patients using the blood NPRL2 mRNA values. Sensitivity (y axis) was plotted against false-positive fraction (1-specificity). The area under receiver operating characteristics curve (AUC) is 0.896. (B) Receiver-operating characteristics curve for healthy and CRC patients using blood NPRL2 mRNA values. Sensitivity (y axis) was plotted against false-positive fraction (1-specificity). The area under receiver operating characteristics curve (AUC) is 0.999. (C) Receiver-operating characteristics curve for colorectal adenoma and CRC patients using blood NPRL2 mRNA values. Sensitivity (y axis) was plotted against false-positive fraction (1-specificity). The area under receiver operating characteristics curve (AUC) is 0.968.
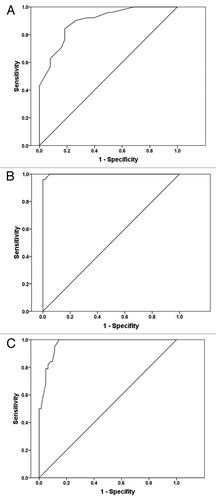
ROC curve analysis showed that blood NPRL2 mRNA levels could discriminate colorectal adenoma and healthy controls. The value of area-under-the-curve (AUC) was 0.896 (95% confidence interval [CI] = 0.832–0.959, P < 0.0001). Based on the best Youden index (the maximum value of [sensitivity + specificity – 1]) for NPRL2, a cut off < 0.955-fold relative to normal control was a positive criterion for the statistical analysis of NPRL2 mRNA in blood.
ROC curve analysis also showed that blood NPRL2 mRNA levels could discriminate CRC and healthy controls with AUC of 0.999 (96.1% sensitivity and 98.4% specificity, P < 0.0001), and colorectal adenoma and CRC with AUC of 0.968 (95% confidence interval [CI] = 0.939–0.996, P < 0.05) (94.7% sensitivity and 88.7% specificity, P < 0.0001).
Discussion
Previous studies have shown that the aberrant expression of NPRL2 in hepatocellular carcinoma makes it a potential independent prognostic tumor marker in this type of cancer.Citation17 Disease-free survival and the overall survival rate of patients with hepatocellular carcinoma who had lower NPRL2 mRNA expression levels were significantly shorter than patients who had higher NPRL2 expression. In addition, recent studies have reported that NPRL2 has a potential to be a biomarker for the prediction of cisplatin response and prognosis in patients with lung cancer, as well as a molecular therapeutic target for enhancing and resensitizing the response of non-responders to cisplatin treatment.Citation22 Furthermore, a recent study by Yogurtcu et al. has shown that decreased NPRL2 mRNA expression may contribute to the progression of colorectal cancer. Indeed, low NPRL2 expression was more frequently observed in poorly differentiated CRC tumors than in the well or moderately differentiated ones.Citation23 Until now, no studies have addressed the expression of NPRL2 in the blood of CRC patients.
Evidence is accumulating that primary cancers begin shedding neoplastic cells in the circulation at an early stage. The detection of circulating tumor cells (CTCs) may prove useful for screening, prognostication and monitoring of response to therapy. However, CTCs may represent only 1 cell among 106 peripheral blood mononuclear cells.Citation24 Therefore some of the studies are aimed at detecting the possible biomarker in the peripheral blood, without the separation of CTCs. Indeed, studies have shown that changes in the mRNA expression of specific genes in peripheral blood monocytes could act as biomarkers for the diagnosis of CRC.Citation24,Citation25 Therefore we have decided to examine the NPRL2 mRNA expression in the peripheral blood of control subjects as well as adenoma and CRC patients.
The results of our study have shown that NPRL2 mRNA expression gradually decreases from the normal mucosa to colorectal adenomas and all the way through colorectal carcinomas. NPRL2 mRNA expression in colorectal tumors decreases with tumor progression and histological grade. These findings were further confirmed by the results of the immunohistochemical analysis which showed that NPRL2 protein expression was lower in poorly differentiated tumors than in well or moderately differentiated tumors.
In this study we also analyzed the NPRL2 mRNA levels in the blood of CRC patients. Our results have shown that blood NPRL2 mRNA levels positively correlated with the NPRL2 mRNA expression in tumor tissue. The most important finding of our study was that the blood NPRL2 mRNA levels were significantly lower in adenoma patients compared with the blood NPRL2 mRNA levels detected in the normal group. This means that blood NPRL2 mRNA levels could potentially be a very useful marker for the early diagnosis of adenoma and CRC.
Although the exact mechanism involved in the inactivation of the NPRL2 gene in human cancers still remains unknown, some studies have suggested that NPRL2 might be involved in the DNA mismatch repair, cell cycle checkpoint signaling, and regulation of apoptosis.Citation16,Citation20,Citation22 A study by Ji et al. has shown that intra-tumoral injection of Ad-NPRL2 vectors significantly suppressed the growth of H1299 and A549 tumor xenografts and inhibited A549 experimental lung metastases in nu/nu mice.Citation21 However, NPRL2 deletions are frequently observed in lung, breast, kidney, and other cancers.Citation12 There is strong evidence for screening the blood NPRL2 expression in populations with high risk of CRC. Therefore a better understanding of the biological function of NPRL2 may result in the development of new strategies for the prevention, early detection, diagnosis and treatment of CRCs.
Finally, our study has revealed that decrease in NPRL2 gene expression is associated with the progression of adenoma to CRC. Our study has also demonstrated that aberrant NPRL2 expression may reflect the malignant potential of tumors and therefore could be used as a potential marker in CRC development and progression.
Patients and Methods
Clinicopathological characteristics of patients
The study included 151 patients referred for colonoscopy at the Second Affiliated Hospital of Harbin Medical University from January 2009 to December 2009. Of these 151 patients, 38 were diagnosed with adenoma (13 male and 25 female, median age 49 y, range 32–69 y) and 62 with CRC (35 male and 27 female, median age 56 y, range 38–76 y). The negative controls used in this study were blood samples and colorectal tissue samples taken from patients with irritable bowel disease which were diagnosed by colorectal endoscopy as well as clinical symptoms (20 males and 31 females) with a median age of 50 y (range, 34–67 y). The clinicopathological characteristics of CRC patients enrolled in this study were as follows: 14 patients with stage IV (any T, any N, M1) tumors (median age, 60 y [range, 36–80 y]; 8 males and 6 females; tumor location: right colon, n = 6; left colon, n = 6; rectum, n = 2), 15 patients with stage III (any T, N1–N2, M0) tumors (median age, 58 [range, 40–69]; 8 males and 7 females; tumor location: right colon, n = 8; left colon, n = 7), 15 patients with stage II (T3–T4, N0, M0) tumors (median age, 59 y [range, 48–79 y]; 9 males and 6 females; tumor location: right colon, n = 7; left colon, n = 6; rectum, n = 2], and 18 patients with stage I (T1–T2, N0, M0) tumors (median age, 48 y [range, 33–62 y]; 10 males and 8 females; tumor location, right colon, n = 6; left colon, n = 9; rectum, n = 2; multiple sites, n = 1) ().
A written informed consent was obtained from all patients included in the study. The procedures were approved by the Ethics Committee of Human Experimentation in China. The patients with adenoma and CRC had complete tumor evaluation by endoscopy or surgery at the time of blood withdrawal and during follow-up. None received neoadjuvant treatment. Clinical disease status was determined according to the American Joint Committee on Cancer/Union International Cancer Control (UICC) tumor-node-metastasis classification and stage groupings.Citation26 All diagnoses of carcinoma and adenoma were confirmed by a qualified pathologist (Y.Z.C.)
Samples
Tissue and plasma specimens were obtained at the time of the endoscopic or surgical treatment. Tissue samples were immediately snap-frozen in liquid nitrogen and stored at −80 °C until further use. In addition tissue samples were fixated in 10% formalin, sliced into 4 to 6 mm pieces, dehydrated in ethanol, embedded in paraffin wax, sectioned (5 μm thick) and stained with hematoxylin and eosin (HE) for histopathological analysis. Blood samples (10 mL) were collected in EDTA-containing tubes and sample processing was performed within 2 h after blood withdrawal. Lymphocytes were isolated using lymphocytes separation medium (LSM, MP Biomedicals) and by centrifugation at 1600 g for 15 min. Following the serum removal the monocyte pellet was resuspended in 0.2 mL TRIzol (Invitrogen) and stored at −80 °C.
Immunohistochemical staining
NPRL2 was detected in paraffin-embedded liver sections using a NPRL2-specific antibody (mouse polyclonal to NPRL2, Abcam, ab88691) and an avidin–biotin complex immunoperoxidase method. After antigen retrieval by immersion in EDTA (1 mmol/L, pH 8.0) and boiling for 15 min in a microwave oven, sections were pre-blocked with normal goat serum for 10 min and incubated for 2 h at room temperature with NPRL2 antibody (final concentration 1:200). Phosphate buffer saline (PBS) was used in place of the primary antibodies for the negative controls. Following rinsing the biotinylated secondary antibody, avidin–biotin complex and horseradish peroxidase (Strept ABComplex; DAKO) were applied. Finally, the sections were washed with PBS, developed with diaminobenzidine tetrahydrochloride substrate for 3 min and counterstained with hematoxylin.
The total NPRL2 immunostaining score was calculated as both the percentage of positively stained cells and the staining intensity as reported in the previous study.Citation27 The percentage of positivity was scored as “0” (<5%, negative), “1” (5–25%, sporadic), “2” (25–50%, focal), or “3” (>50%, diffuse). The staining intensity was scored as “0” (no staining), “1” (weakly stained), “2” (moderately stained), or “3” (strongly stained). The immunostaining scores was evaluated by two pathologists and calculated as the percentage positive score × the staining intensity score and it ranged from 0 to 9. The NPRL2 expression levels were as follows: “−” (score 0–1), “+” (score 2–3), “++” (score 4–6), and “+++” (score >6). Based on the NPRL2 expression levels, the CRC patients were divided into two groups: the low NPRL2 expression group (NPRL2− or NPRL2+) and the high NPRL2 expression group (NPRL2++ or NPRL2+++).
Expression of NPRL2 mRNA transcripts
Total RNA was extracted from monocytes using Trizol (Invitrogen). The integrity of RNA was evaluated by visualizing the 18S and 28S RNA on agarose gels. Then RNA was reverse transcribed into cDNA using the Rnase reverse transcriptase assay (Fermentas) in a final volume of 150 μL according to the manufacturer’s instructions. CDNA was used to quantify the expression of NPRL2 mRNA by real-time polymerase chain reaction (RT-PCR) on an ABI 7500 instrument (Applied Biosystems). PCR was performed in 20 μL reaction mixture containing the 2 μg of cDNA, 1 μL of each primer, and 10 μL of SYBR Green PCR Master Mix (Applied Biosystems). The NPRL2 mRNA expression was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression in each analyzed sample. The sequences of the primers used are as follows: GADPH, F: 5′-CCTGCCAAGT ATGATGACAT CAAGA-3′, R: 5′-GTAGCCCAGG ATGCCCTTTA GT-3′; NPRL2, F: 5′-GGACCTCACT ACACAACAAA TCCTG-3′, R: 5′-GTCACAACGC CGTAGTACAG CA-3′.
Statistical analysis
The results are presented as the mean ± standard deviation (SD). Statistical tests were performed with SPSS software version 11.5 (SPSS Inc.). The difference between the two groups was analyzed using a Student t test and comparisons between more than 2 groups were analyzed by one-way analysis of variance (ANOVA). The χ2 test for proportion was used to analyze the relationship between the NPRL2 expression and various pathological characteristics. P values lower than 0.05 were considered as statistically significant.
| Abbreviations: | ||
| NPRL2 | = | nitrogen permease regulator-like 2 |
| CRC | = | colorectal cancer |
| PBS | = | phosphate buffer saline |
| TSG | = | intensive tumor suppressor gene |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60:277 - 300; http://dx.doi.org/10.3322/caac.20073; PMID: 20610543
- Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer 2010; 46:765 - 81; http://dx.doi.org/10.1016/j.ejca.2009.12.014; PMID: 20116997
- Jung KW, Park S, Kong HJ, Won YJ, Boo YK, Shin HR, Park EC, Lee JS. Cancer statistics in Korea: incidence, mortality and survival in 2006-2007. J Korean Med Sci 2010; 25:1113 - 21; http://dx.doi.org/10.3346/jkms.2010.25.8.1113; PMID: 20676319
- Chen HM, Weng YR, Jiang B, Sheng JQ, Zheng P, Yu CG, Fang JY. Epidemiological study of colorectal adenoma and cancer in symptomatic patients in China between 1990 and 2009. J Dig Dis 2011; 12:371 - 8; http://dx.doi.org/10.1111/j.1751-2980.2011.00531.x; PMID: 21955430
- Rougier P, Mitry E. Epidemiology, treatment and chemoprevention in colorectal cancer. Ann Oncol 2003; 14:Suppl 2 ii3 - 5; http://dx.doi.org/10.1093/annonc/mdg722; PMID: 12810450
- Luo Y, Wang L, Wang J. Developing proteomics-based biomarkers for colorectal neoplasms for clinical practice: opportunities and challenges. Proteomics Clin Appl 2013; 7:30 - 41; http://dx.doi.org/10.1002/prca.201200071; PMID: 23255431
- Andersen V, Holst R, Vogel U. Systematic review: diet-gene interactions and the risk of colorectal cancer. Aliment Pharmacol Ther 2013; 37:383 - 91; http://dx.doi.org/10.1111/apt.12180; PMID: 23216531
- Bretthauer M, Kalager M. Principles, effectiveness and caveats in screening for cancer. Br J Surg 2013; 100:55 - 65; http://dx.doi.org/10.1002/bjs.8995; PMID: 23212620
- Sillars-Hardebol AH, Carvalho B, van Engeland M, Fijneman RJ, Meijer GA. The adenoma hunt in colorectal cancer screening: defining the target. J Pathol 2012; 226:1 - 6; http://dx.doi.org/10.1002/path.3012; PMID: 21984228
- Creeden J, Junker F, Vogel-Ziebolz S, Rex D. Serum tests for colorectal cancer screening. Mol Diagn Ther 2011; 15:129 - 41; http://dx.doi.org/10.1007/BF03256403; PMID: 21766904
- Church D, Midgley R, Kerr D. Biomarkers in early-stage colorectal cancer: ready for prime time?. Dig Dis 2012; 30:Suppl 2 27 - 33; http://dx.doi.org/10.1159/000341890; PMID: 23207929
- Sclafani F, Gullo G, Sheahan K, Crown J. BRAF mutations in melanoma and colorectal cancer: a single oncogenic mutation with different tumour phenotypes and clinical implications. Crit Rev Oncol Hematol 2013; 87:55 - 68; http://dx.doi.org/10.1016/j.critrevonc.2012.11.003; PMID: 23246082
- Terrin L, Rampazzo E, Pucciarelli S, Agostini M, Bertorelle R, Esposito G, DelBianco P, Nitti D, De Rossi A. Relationship between tumor and plasma levels of hTERT mRNA in patients with colorectal cancer: implications for monitoring of neoplastic disease. Clin Cancer Res 2008; 14:7444 - 51; http://dx.doi.org/10.1158/1078-0432.CCR-08-0478; PMID: 19010861
- Wheeler JM. Epigenetics, mismatch repair genes and colorectal cancer. Ann R Coll Surg Engl 2005; 87:15 - 20; http://dx.doi.org/10.1308/1478708051423; PMID: 15720901
- Lerman MI, Minna JD. The 630-kb lung cancer homozygous deletion region on human chromosome 3p21.3: identification and evaluation of the resident candidate tumor suppressor genes. The International Lung Cancer Chromosome 3p21.3 Tumor Suppressor Gene Consortium. Cancer Res 2000; 60:6116 - 33; PMID: 11085536
- Li J, Wang F, Haraldson K, Protopopov A, Duh FM, Geil L, Kuzmin I, Minna JD, Stanbridge E, Braga E, et al. Functional characterization of the candidate tumor suppressor gene NPRL2/G21 located in 3p21.3C. Cancer Res 2004; 64:6438 - 43; http://dx.doi.org/10.1158/0008-5472.CAN-03-3869; PMID: 15374952
- Otani S, Takeda S, Yamada S, Sakakima Y, Sugimoto H, Nomoto S, Kasuya H, Kanazumi N, Nagasaka T, Nakao A. The tumor suppressor NPRL2 in hepatocellular carcinoma plays an important role in progression and can be served as an independent prognostic factor. J Surg Oncol 2009; 100:358 - 63; http://dx.doi.org/10.1002/jso.21241; PMID: 19274676
- Yi Lo PH, Chung Leung AC, Xiong W, Law S, Duh FM, Lerman MI, Stanbridge EJ, Lung ML. Expression of candidate chromosome 3p21.3 tumor suppressor genes and down-regulation of BLU in some esophageal squamous cell carcinomas. Cancer Lett 2006; 234:184 - 92; http://dx.doi.org/10.1016/j.canlet.2005.03.036; PMID: 15885884
- Jayachandran G, Ueda K, Wang B, Roth JA, Ji L. NPRL2 sensitizes human non-small cell lung cancer (NSCLC) cells to cisplatin treatment by regulating key components in the DNA repair pathway. PLoS One 2010; 5:e11994; http://dx.doi.org/10.1371/journal.pone.0011994; PMID: 20700484
- Ueda K, Kawashima H, Ohtani S, Deng WG, Ravoori M, Bankson J, Gao B, Girard L, Minna JD, Roth JA, et al. The 3p21.3 tumor suppressor NPRL2 plays an important role in cisplatin-induced resistance in human non-small-cell lung cancer cells. Cancer Res 2006; 66:9682 - 90; http://dx.doi.org/10.1158/0008-5472.CAN-06-1483; PMID: 17018626
- Ji L, Nishizaki M, Gao B, Burbee D, Kondo M, Kamibayashi C, Xu K, Yen N, Atkinson EN, Fang B, et al. Expression of several genes in the human chromosome 3p21.3 homozygous deletion region by an adenovirus vector results in tumor suppressor activities in vitro and in vivo. Cancer Res 2002; 62:2715 - 20; PMID: 11980673
- Schenk PW, Brok M, Boersma AW, Brandsma JA, Den Dulk H, Burger H, Stoter G, Brouwer J, Nooter K. Anticancer drug resistance induced by disruption of the Saccharomyces cerevisiae NPR2 gene: a novel component involved in cisplatin- and doxorubicin-provoked cell kill. Mol Pharmacol 2003; 64:259 - 68; http://dx.doi.org/10.1124/mol.64.2.259; PMID: 12869630
- Yogurtcu B, Hatemi I, Aydin I, Buyru N. NPRL2 gene expression in the progression of colon tumors. Genet Mol Res 2012; 11:4810 - 6; http://dx.doi.org/10.4238/2012.September.12.3; PMID: 23079973
- Xi L, Nicastri DG, El-Hefnawy T, Hughes SJ, Luketich JD, Godfrey TE. Optimal markers for real-time quantitative reverse transcription PCR detection of circulating tumor cells from melanoma, breast, colon, esophageal, head and neck, and lung cancers. Clin Chem 2007; 53:1206 - 15; http://dx.doi.org/10.1373/clinchem.2006.081828; PMID: 17525108
- Guadagni F, Kantor J, Aloe S, Carone MD, Spila A, D’Alessandro R, Abbolito MR, Cosimelli M, Graziano F, Carboni F, et al. Detection of blood-borne cells in colorectal cancer patients by nested reverse transcription-polymerase chain reaction for carcinoembryonic antigen messenger RNA: longitudinal analyses and demonstration of its potential importance as an adjunct to multiple serum markers. Cancer Res 2001; 61:2523 - 32; PMID: 11289125
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17:1471 - 4; http://dx.doi.org/10.1245/s10434-010-0985-4; PMID: 20180029
- Yang XB, Zhao JJ, Huang CY, Wang QJ, Pan K, Wang DD, Pan QZ, Jiang SS, Lv L, Gao X, et al. Decreased expression of the FOXO3a gene is associated with poor prognosis in primary gastric adenocarcinoma patients. PLoS One 2013; 8:e78158; http://dx.doi.org/10.1371/journal.pone.0078158; PMID: 24194912
