Abstract
Pseudoxanthoma elasticum (PXE) is a heritable disease characterized by calcified elastic fibers in cutaneous, ocular, and vascular tissues. PXE is caused by mutations in ABCC6, which encodes a protein of the ATP-driven organic anion transporter family. The inability of this transporter to secrete its substrate into the circulation is the likely cause of PXE. Vitamin K plays a role in the regulation of mineralization processes as a co-factor in the carboxylation of calcification inhibitors such as Matrix Gla Protein (MGP). Vitamin K precursor or a conjugated form has been proposed as potential substrate(s) for ABCC6. We investigated whether an enriched diet of vitamin K1 or vitamin K2 (MK4) could stop or slow the disease progression in Abcc6-/- mice. Abcc6-/- mice were placed on a diet of either vitamin K1 or MK4 at 5 or 100 mg/kg at prenatal, 3 weeks or 3 months of age. Disease progression was quantified by measuring the calcium content of one side of the mouse muzzle skin and histological staining for calcium of the opposing side. Raising the vitamin K1 or MK4 content of the diet increased the concentration of circulating MK4 in the serum. However, this increase did not significantly affect the MGP carboxylation status or reduce its abnormal abundance, the total calcium content or the pathologic calcification in the whiskers of the 3 treatment groups compared to controls. Our findings showed that raising the dietary intake of vitamin K1 or MK4 was not beneficial in the treatment of PXE and suggested that the availability of vitamin K may not be a limiting factor in this pathology.
Introduction
Pseudoxanthoma elasticum (PXE, OMIM 264800) in humans is defined by dystrophic and progressive mineralization of elastic fibers in cutaneous, ocular and vascular tissues. This condition derives from loss-of-function mutations in the human ABCC6 and mouse Abcc6 genes (alias Multidrug Resistance Protein 6 or MRP6).Citation1–Citation3 ABCC6 is primarily expressed in liver and has a much reduced but measurable expression in kidney, intestine and the retina. To a much lesser extent, ABCC6 is detectable in many other tissues including lung, skin and vessel walls.Citation4–Citation7 ABCC6 is a member of the large ATP-binding cassette (ABC) gene subfamily C, which uses ATP hydrolysis to drive organic anion transport across cellular membranes. ABCC6 actively transports glutathione conjugates,Citation7 but as yet, the specific substrate for this transporter, and hence the contributing factor to PXE, remains unknown. Importantly, the encoded transmembrane protein is located in the basolateral membrane of hepatic cells and the basal lateral membrane of the proximal kidney tubules where the inability of this transporter to secrete its unknown substrate towards the circulation is hypothesized to be the cause of the PXE disease progression and describes PXE as a metabolic disorder.Citation8
Control of bone mineralization and calcification of soft tissues is a highly regulated process, requiring a fine balance between inhibitors and promoters at both the circulatory and local level.Citation9,Citation10 Among the primary local regulators of mineralization, Matrix Gla Protein (MGP) and osteocalcin (OC) are reliant on carboxylation for their activation and function.Citation10,Citation11 Gheduzzi et al. described PXE patients with a lower serum concentration of MGP and a higher ratio of under carboxylated MGP than in controls. In addition, this under carboxylated MGP co-localized with calcified elastic fibers.Citation10 Uitto et al. also found the connective tissue capsule surrounding the vibrissae of the Abcc6-/- mouse whisker was calcified and contained exclusively under carboxylated MGP.Citation12 Vitamin K is an essential component in the post-translational carboxylation of glutamate residues (Glu) in these inhibitor proteins. The reduced form of vitamin K acts as cofactor in the conversion of the Glu (inactive) form to the Gla form (γ-carboxyglutamate). With this in mind, it was recently hypothesized that a vitamin K precursor or a conjugated form was the potential substrate(s) for ABCC6.Citation13
It is also known that PXE patients have low serum vitamin K levels and it was proposed that this low level might result in insufficient carboxylation of the circulating inhibitors of mineralization thus leading to mineral deposition in peripheral tissues. A subgroup of mutations in the gamma-glutamyl carboxylase—gene (GGCX) that encodes an enzyme essential in the carboxylation of Gla proteins results in a PXE-like phenotype and some coagulation factor deficiency. Mutations in the vitamin K 2,3 epoxide reductase (VKORC1) however, only result in deficiencies in the vitamin K dependent clotting factors (II, VII, IX, X).Citation14 The link between vitamin K, vitamin K-dependent (VKD) proteins and PXE or PXE-like phenotypes has been previously discussed in references Citation15 and Citation16.
Vitamin K is a group of lipophilic, hydrophobic compounds. Of the two forms of naturally occurring vitamin K, vitamin K1- phylloquinone (phytomenadione), is the primary source for humans. Obtained from the leafy green plants and vegetables, it is poorly absorbed but has a high hepatic concentration. Vitamin K2 or menaquinones, are more unsaturated and have a variable number of isoprenyl groups (four in the case of MK4). There is an increased concentration of the menaquinones in extra-hepatic tissue compared to vitamin K1. MK4 is synthesized by mammalian tissues and additionally converted from vitamin K1.Citation17,Citation18 Some functions of the menaquinones and vitamin K1 appear interchangeable, notably in the activation of clotting factors but others such as bone resorption are more specific to the particular chemical structure of the type of vitamin K. However, one should note that depending on the type of vitamin K being tested, either phylloquinone or menaquinone, the efficacy of these compounds seems to depend on the specific circumstances being investigated.Citation19–Citation21 Of particular relevance to this investigation are studies using a rat model of arterial calcification. Here vitamin K1 and vitamin K2 (MK4) were shown to be equally effective in reversing the warfarin-induced calcification, thus implying interchangeable roles for these two compounds within this particular model.Citation22
The phenotype of the Abcc6-/- mice we used has previously been described in reference Citation23. Two Abcc6 knockout mice essentially recapitulate the PXE disease progression with mineralization of the skin, the Bruch's membrane of the eye and small size arteries.Citation23,Citation24 One notable feature of these mice, and of particular interest in this study, is the calcification of the vibrissae's capsule in the whiskers, the connective tissue capsule surrounding the hair follicle.Citation23,Citation24 The calcification of this tissue (not present in humans) occurs at approximately 5 weeks of age and is reported to be progressiveCitation23 and quantifiable and thus serves as a marker of disease progression.Citation25
To address the hypothesis that a vitamin-K dependent calcification inhibition process plays a role in the pathology of PXE,Citation15,Citation16 we investigate whether a high dietary intake of vitamin K could be beneficial to PXE patients. For this purpose, we used an Abcc6-/- mouse model of the disease.Citation23 We aimed to discover whether the provision of a diet enriched with two active forms of vitamin K (K1 and MK4) could compensate any negative effects of the Abcc6 knockout on the development of mineralization. Secondly, we investigated whether the lack of Abcc6 had any effect on the absorption, metabolism or distribution of vitamin K compared to WT controls under normal diet conditions.
Results
Progression of the PXE phenotype.
In order to determine the optimum time range to conduct our experiments to stop or minimize the progression of calcification, we precisely quantified the mineralization of the whisker over the life span of Abcc6-/- mice (approximately 28–32 months). We established that within the period from 3 weeks to approximately 6 months of age there is a near-exponential rate of deposition of calcium in the vibrissae. This rate of calcification slows after 6 months of age and reaches a gently sloping plateau thereafter up to the natural demise of the animal ().
Effects of vitamin K supplementation.
The diet schedule of 5 mg (low dose) or 100 mg/kg (high dose) vitamin K1 or vitamin MK4 corresponded to a 5-fold and 100-fold increase respectively in vitamin K1 above normal diet. For the MK4 supplementation, the actual increase could only estimated to be in the similar range of 5 to 100-fold because this form of vitamin K2 can be readily converted in vivo from vitamin K1, which is naturally present as traces in the animal chow and can also be synthesized by extra-hepatic tissues.Citation26 However, given the large increase in serum concentration of MK4 in mice fed the MK4 enriched diet, the diet supplementation in vitamin MK4 produced the expected boost in the circulation.
Interestingly, mice fed with both concentrations of the enriched vitamin K1 diet showed no overt adverse effects on physiology or behavior, whereas Abcc6-/- mice fed with a high dose of MK4 had marked liver discoloration, with small distinct lesions on the liver surface. These mice also showed an apparent increased propensity to blood clotting observed upon blood withdrawal. Approximately 50% of the WT mice fed the enriched high dose MK4 diet also had areas of hair loss and skin lesions consistent with increased scratching. However, no change in the gross appearance of their liver was observed.
Vitamin K1 or MK4 enriched diets do not affect tissue mineralization in Abcc6-/- mice.
Neither of the vitamin K enriched diets or treatment times had any effect on the total calcium content of the vibrissae tissue for WT mice, which contained very low levels of calcium ( and ). In contrast to WT mice, Abcc6-/- mice fed with the normal diet up to 5 months of age had significantly elevated total calcium content levels in the vibrissae area. Importantly, we measured no significant change in the total calcium content of the vibrissae between Abcc6-/- mice fed on a normal diet or the vitamin K1 enriched diet for any of the three treatment groups (in utero, from pre-onset or from post-onset of phenotype). In each case, we measured significant increases in calcium deposition between WT and Abcc6-/- mice (). Additionally, we measured no significant difference in total calcium content in muzzle tissue taken from Abcc6-/- mice fed exclusively on a normal diet or fed with the high or low dose MK4 enriched diet. In all the MK4 dietary regimes tested, we saw significant increases in total calcium content of the muzzle tissue in Abcc6-/- mice compared with WT ().
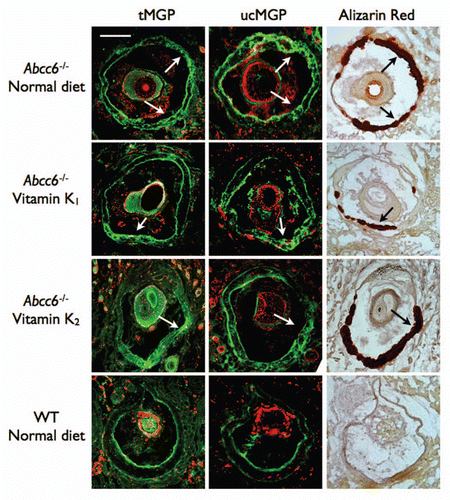
To confirm the location and extent of the calcium deposition within the whiskers, we performed Alizarin red S staining on paraffin sections of the tissue. As expected, no staining was observed in WT mice fed with either the normal or vitamin K enriched diets. In contrast, Abcc6-/- mice fed on a normal diet had significantly elevated Alizarin red staining in the vibrissae consistent with calcium deposition within the capsule. Moreover, no change in the amount or location of the calcium deposition between the normal diet and any of the enriched diet fed mice was observed (). The histological staining was consistent with the calcification quantifications indicating that oral administration of high dosage of either vitamin K1 or vitamin MK4 has no effect on mineral deposition in the vibrissae of an Abcc6-/- mouse.
A diet enriched in vitamin K1 lead to elevated circulating levels of vitamin K1 and MK4.
Using HPLC-MS, we analyzed circulating levels of vitamin K1 and MK4 in the serum of mice fed with either a normal diet, or vitamin K1 or vitamin MK4 low and high dose enriched diets. Baseline vitamin K1 serum content was defined from WT mice fed a normal diet. In both the control WT pre-onset and 3 months feeding groups fed with a high dose of vitamin K1, we detected a significant increase in vitamin K1 levels above baseline. However, we were unable to detect significant increases in vitamin K1 from WT mice fed on the low dose diets, thus indicating that 5 mg/kg was ineffective at significantly raising the concentration of circulating vitamin K1 in these animals (). Although we saw some increase in the levels of vitamin K1 circulating in the serum of Abcc6-/- mice fed with the high dose, 100 mg/kg, vitamin K1 at pre-onset and the in utero group, it was not a statistically significant increase (). Moreover, in comparison to paired WT controls, the Abcc6-/- mice in the pre-onset group fed the high dose diet had significantly less vitamin K1 circulating thus indicating that Abcc6 could play some role in vitamin K1 absorption or circulation ().
Our studies also showed interesting differences in the levels of circulating MK4 in mice fed exclusively with normal or vitamin K1 enriched diets. In mice fed a normal diet, we measured significantly more circulating MK4 in WT mice than in Abcc6-/-. Similarly in mice fed the high dose vitamin K1 diet, WT mice had significantly more circulating MK4 than Abcc6-/- mice (). In general, in Abcc6-/- mice fed the enriched vitamin K1 diet, we were able to measure an increase in circulating MK4 compared to Abcc6-/- mice fed on a normal diet. However, this increase was not statistically significant ().
A vitamin MK4-enriched diet boost MK4 circulating levels.
Serum analysis from WT mice fed on a diet enriched with 5 mg/kg of vitamin MK4 showed significantly increased levels of this compound indicating that this dosage was quite effective in raising the serum levels of MK4. In Abcc6-/- mice however, the increase in circulating MK4 from mice fed on the low dose MK4 diet was not significant. In contrast, the Abcc6-/- mice fed with the high dose of MK4 in both the pre- and post-onset groups had significantly increased levels of circulating MK4 compared to WT and Abcc6-/- mice fed a normal diet. It is important to note that this data indicates that in Abcc6-/- mice, MK4 was available to the periphery in quantities greater than seen in WT or Abcc6-/- animals on a normal diet (). Also, we detected very low levels of circulating MK4 in the Abcc6-/- in utero group fed with the high, 100 mg/kg, vitamin K2 diet and at present we are unable to explain this result, though one may suggest an adaptation of the mice to a greater influx of the vitamin from the diet over a longer period of time.
The carboxylation status of MGP and OC in the capsule of the vibrissae.
We found no change in the carboxylation status of MGP in the capsule surrounding the vibrissae. The ucMGP was found mainly associated with the calcified area. MGP detected with an antibody that targets both under-carboxylated and carboxylated MGP was found also throughout the calcified regions of the capsule. We found no major association between OC and the calcification in the vibrissae of Abcc6-/- mice. Furthermore, the diet, whether normal or enriched with vitamin K, produced no visible change in OC levels in the vibrissae's capsule (data not shown).
Blood chemistry.
We found no significant difference in the blood chemistry between mice fed either a normal diet or the vitamin K1 5 or 100 mg/kg diets for all parameters. Ion levels, blood glucose, pH etc., were all within normal physiological levels as seen with the control normal diet fed mice (data not shown).
Discussion
Mutations in ABCC6 alone cannot explain the high phenotypic variation in PXE patients.Citation27,Citation28 More emphasis is now being placed on environmental factors such as: diet, smoking and hormone balance to explain the complexity of the intra/inter familial phenotypic variation.Citation29,Citation30 Although PXE patients have no deficiency in the vitamin K-dependent clotting factors synthesized within the liver, which incidentally suggest an adequate supply of vitamin K in this tissue, they have very low concentrations of this vitamin in the periphery.Citation16 Additional work by Vanakker et al. established a strong link between low serum concentration of vitamin K and decreased carboxylation of inhibitors of the mineralization process in the plasma and serum such as MGP and OC.Citation16 Studies in rats have also shown that supplementation with vitamin K can reverse arterial calcification induced by warfarin, a known inhibitor of the vitamin K cycle. This is probably due to an increase in carboxylated MGP levels at the site of the calcium deposit.Citation22 This and the fact that PXE is a metabolic disease, and that a PXE-like disease with a similar phenotype is related to vitamin K, has led to the hypothesis of ABCC6 as a transporter of vitamin K.Citation13 The aim of this study was therefore to investigate whether a large increase in vitamin K from the diet could raise the peripheral availability of vitamin K and thus have an effect on the calcification process in Abcc6-/- mice, an animal model for PXE.
The link between vitamin K and mineralization is now well established,Citation31 but due to the nature of vitamin K being a family of chemically related compounds with differing chemical structures, their respective activities are not necessarily fully understood. The type and dosage of vitamin K can also have vastly different effects on the model being studied. For example the Rotterdam study, a prospective, population-based cohort study, has shown that menaquinone but not phylloquinone is important in preventing vascular calcification and coronary heart disease.Citation32 However, studies in rats using plasma prothrombin levels as a marker for vitamin K status have shown that low dietary doses of phylloquinone is more effective at maintaining vitamin K status than menaquinone (MK9), but at higher doses the activity is the same.Citation21 For this reason we designed our experiments using two types of commercially available vitamin K (K1 and MK4) to increase the probability of measuring an effect of this vitamin on the disease progression.
One advantage of using the Abcc6-/- mouse model is the consistency of its biomarkers of disease progression. The level of calcification in the bulb of the vibrissae between the Abcc6-/- mice is very consistent and also easily measurable. When compared with staining typically observed in Abcc6-/- mice fed with a control diet, we found no alteration in the staining patterns around the bulb of the vibrissae in any of the vitamin K treatment groups (). Analyzing the total calcium content of the muzzle tissue negates the need for time-consuming morphometric analysis of stained tissue sections and additionally removes any bias introduced by choice and depth of sections to analyze. This method is very sensitive as we were able to reliably detect a significant increase in amount of calcification between groups of Abcc6-/- mice aged 4 weeks apart. Using total calcium quantification, we confirmed our findings initially obtained with Alizarin Red S staining and found no effect of any of the feeding regimes of either vitamin K1 or MK4 on total calcium content of the muzzle tissue in Abcc6-/- mice ( and ). In addition, we compared kidney tissue sections from vitamin K treated Abcc6-/- mice and found typical levels of calcification (data not shown).Citation23,Citation33
Because of the lack of effect of vitamin K supplementation on the progression and severity of the mineralization phenotype of Abcc6-/- mice, we verified how much of the dietary increase in vitamin K1 and MK4 was actually absorbed, passed into the circulation and was available to peripheral tissues. The reduction in circulating vitamin K1 levels measured in human PXE patientsCitation16 was not reproduced in our mouse model. We measured no significant difference () in the serum baseline concentration of circulating vitamin K1 in the Abcc6-/- and WT animals fed with a normal diet, with both strains of mice having a low concentration of this vitamin K form and therefore providing the first indication that a reduced peripheral vitamin K1 concentration is not a factor in the mouse mineralization phenotype. However, we did measure a significantly lower baseline circulating level of MK4 in the Abcc6-/- mice compared to WT. This not only indicates less availability of this form of vitamin K for carboxylase function at the periphery, but also suggests a lower rate of conversion from vitamin K1 in the knockout animals. Both WT and Abcc6-/- mice fed with high dose vitamin K1 diet had increased levels of both vitamin K1 and MK4 ( and ), indicating that the conversion of MK4 from vitamin K1 followed the increase in the availability of phylloquinone. However, although the high vitamin K1 diet induced a significant increase in vitamin K1 serum concentration in Abcc6-/- mice, the increase was significantly less than the equivalent serum K1 levels in control WT mice (). This discrepancy in circulating serum K1 levels between the two strains indicates that in Abcc6-/- mice the vitamin K1 is absorbed from the chow and re-circulated into the blood stream but with less efficiency than in WT.
In our mice treated with the high dose diet of MK4, we measured no difference between circulating MK4 in WT or Abcc6-/- mice indicating a good absorbance of this form of vitamin K from the diet. Additionally, these data show that elevated levels of vitamin K1 and MK4 were effectively absorbed from the diet and are able to pass into the circulation in WT and Abcc6-/- animals, and thus potentially available to peripheral tissues for the activation of MGP by carboxylation. We detected an increase in total MGP in the vibrissae of Abcc6-/- mice in association with areas of mineralization as expected.Citation25 However, large proportion of this MGP remained un-carboxylated despite the increase in the availability of the vitamin K co-factors. The increased level of total MGP in association with calcified regions in the whiskers, compared to the levels observed in the WT mice, suggested a heightened synthesis of MGP perhaps as a compensatory mechanism. Nevertheless, this increase appears ineffectual in overcoming the calcium deposition because the vast majority of this overproduced MGP is not carboxylated.
This could imply several possibilities: first, the amount of circulating vitamin K is not a factor in determining how much diffuses from the blood and is available in the peripheral tissues for the carboxylation processes. Second, assuming the contrary, that the increased vitamin K is indeed available to the peripheral tissues, the amount of vitamin K does not determine the rate of the carboxylation of the VKD inhibitors of mineralization in these mice. Third, the increased amount of MGP observed in the calcified regions of the vibrissae is not concomitant to an increase in carboxylation activity by GGCX. Or lastly, MGP is not a major player in the pathogenesis of mineralization in this model, though this possibility appears less likely. Either way, the hypothesis that the “absence of ABCC6 in PXE prevents the liver from providing sufficient Vitamin K to the periphery” cannot be proven in our model.
Based on the discrepancy in MK4 serum levels between WT and Abcc6-/- mice fed on a normal diet and the lack of increase of MK4 serum levels in the vitamin K1 fed Abcc6-/- mice, which was most likely due to less K1 being available for conversion to MK4 (), we suggest that Abcc6 might be involved in vitamin K transport, absorption or metabolism in the body, but only to a limited degree. This would be in agreement with Vanaker et al. (2010).Citation16 Along with the fact that the livers of the Abcc6-/- mice on the highest vitamin MK4 dose (but not the livers of the WT animals) were discolored and had an altered physical appearance leads us to infer that a reduced amount of vitamin K reaches the periphery, with a potential hepatic bottle neck. It is known that Abcc6 is present in the gut.Citation33,Citation34 If the transport from the gut is adversely affected by the loss of Abcc6 and therefore isn't fully absorbed, and/or additional transport from the liver into the peripheral tissue would also be reduced leading to the observed changes in liver appearance. This hypothesis could be relatively easily explored using other routes of delivery to raise the vitamin K levels in the periphery, such as intravenous administration, but due to our results showing that inducing an increase in MK4 is possible in the Abcc6-/- mice (however not effective in treating the disease progression), it is not easily rationalized.
In summary, the results of the present study and those from Vanakker et al. (2010)Citation16 both suggest a role of Abcc6 in vitamin K absorption, distribution or metabolism. However, our data also provided conclusive evidence that a dietary supplementation in vitamin K is not a viable approach to preventing PXE-related calcification thus suggesting that the availability of vitamin K is not a limiting factor in the pathology of PXE and/or that vitamin K-dependent inhibition of calcification may not be as preponderant in the development of this disease as previously thought.
Materials and Methods
Animals.
C57BL/6J mice designated here as wild-type (WT) controls were purchased from Jackson laboratories. Abcc6-/- mice were obtained from the laboratory of Dr. J. Uitto at the Thomas Jefferson UniversityCitation23 via a standard UBMTA. Mice used in these study have been housed and cared for in an approved Animal Care facility in the BioScience Building of the University of Hawaii School of Medicine. The mice were kept under routine laboratory conditions with 12 hours light-dark cycle with access ad libitum to water and standard chow. The Institutional Animal Care and Use Committee of the University of Hawaii approved this study. Experiments have been conducted according to national guidelines.
Diet.
Abcc6-/- and WT mice were placed on a diet enriched with either 5 (low dose) or 100 mg/kg (high dose) of vitamin K1 (phylloquinone) or vitamin K2, specifically MK4. The specifically formulated diets were purchased from Bio-Serv, Inc., (AIN-93M). We designed our vitamin K-enriched diet experiment to take place within the first 5 months of life during the phase of maximal mineralization and to cover several treatment options for different developmental ages: (1) Vitamin K-enriched feeding from in utero to 4.5 months aimed to prevent calcification with the maximum time of increased vitamin K exposure. These mice were exposed to vitamin K in utero, breast-fed from vitamin K-fed mothers and then weaned on enriched vitamin K diets. Next, (2) by treating mice from weaning age at 3 weeks, which is before the onset of the phenotype (pre-onset), to 4.5 months old we sought to test whether the mineralization phenotype could be averted by exposing young mice to the diets. Finally, (3) mice were fed from 3 months of age, which is after the onset of the phenotype (post-onset) for 12 weeks. The latter experiment was designed to test whether treatment with the vitamin K diets could stop or possibly reverse calcification progression. This particular treatment regime is likely the most applicable as a potential treatment to the PXE condition since diagnosis is usually made after the phenotype has occurred.
Histochemisty and immunohistochemistry.
Direct histological visualization of calcium deposition following Alizarin red S staining on paraffin-embedded sections was carried out on the left muzzle skin of each mouse. Briefly, 5 µm slides were deparaffinized and incubated in Alizarin red S solution (Sigma) for 5 minutes, excess dye was removed with a 50% mixture of acetone and xylene and the slides were mounted with Permount (Fisher Scientific). Selected sections from each treatment group were examined for calcium deposition and images were collected using an Axioscope 2 fluorescent microscope.
For immunofluorescent staining, muzzle skin samples were quickly harvested, placed in Optimum Cutting Temperature (OCT) compound and stored at −80°C. Immunofluorescent staining of whisker samples from high dose diets was performed using 5 µm-thick frozen sections. A mouse monoclonal anti-ucMGP antibody (VitaK BV, Maastricht) was used to specifically detect the uncarboxylated MGP. A rabbit polyclonal anti-tMGP antibody (Santa Cruz biotech, Santa Cruz, CA) was used identify total MGP (carboxylated and uncarboxylated) while OC was visualized with a rabbit polyclonal antibody (Millipore, Temecula, CA). The secondary antibody was Alexafluor 488 (Invitrogen, Carlsbad, CA). The distribution of MGP and OC in the capsule of the vibrissae was determined by imaging using an Axioscope 2 fluorescent microscope (Zeiss, Thornwood, NY). Individual images were collected and processed with Photoshop CS3 (Adobe, San Jose, CA).
Colorimetric measurement.
To quantify the levels of mineral deposition in the different groups of mice we carried out a colorimetric assayCitation35 that measures directly the amount of calcium within excised muzzle tissue, which is then corrected for tissue weight. Briefly, the entire skin from one side of the muzzle was harvested, minced and incubated at room temperature for 48 hours in 0.15 N HCl. The total calcium content of the HCl supernatant was assessed by measuring the absorbance at 550 nm using the Calcium (CPC) Liquicolor kit (Stanbio). Calcium content was normalized to total tissue dry weight prior to mincing. The obtained absorbance values were quantified against a known standard to provide calcium concentration in mg per deciliter and per gram of tissue.
Blood analysis.
Blood samples were harvested from each mouse and the serum was immediately separated by low-speed centrifugation and stored at −80°C until HPLC-MS analysis of total serum vitamin K1 and vitamin MK4 content could be performed. Vitamin K1 and K2 were extracted as described in Spronk et al. (2003),Citation36 with some modifications. Briefly: 25 µl of plasma sample was diluted 1:1 with PBS. For deproteination, 200 µl ethanol was added and vigorously vortexed. Vitamin Ks were extracted with 600 µl n-hexane. After one minute vortexing the samples were centrifuged (5 min at 1,000 rpm) and the hexane-phase was separated and dried under a stream of nitrogen. The extracted material was dissolved in 50 µl isopropanol, and kept at −20°C until HPLC-MS analysis in sealed glass vials. For calibration purposes 0, 1, 5, 25 and 125 nM vitamin K1 and vitamin K2 was added to WT mouse serum and the extraction was carried out in the same way.
Mass spectrometric measurements were run on an AB Sciex 3200 QTrap tandem mass spectrometer. The components were ionized in negative ion mode using an atmospheric pressure chemical ionization (APCI) source. The instrument was scanned in multiple reaction-monitoring (MRM) mode. The MRM transition was 444/185 for MK4 and 450/185 for vitamin K1. The samples were separated prior to mass spectrometric analysis using a Perkin Elmer HPLC system. Mobile phases were: water and acetonitrile with a flow rate of 0.4 ml/min. A Phenomenex LUNA Phenyl-hexyl column (C18, 55 × 2 mm, 3 µm particle size) was used for the separation. The oven temperature was kept at 40°C.
Additionally, analysis of blood chemistry of treated and control mice was performed using the i-STAT EC8+ system (Abbott).
Statistical analysis.
Data were analyzed with the statistical software PRISM® (GraphPad). Statistical analyses were performed using two-tailed unpaired Student's t-tests to determine statistical difference between WT and Abcc6-/- groups. Results were expressed as mean ± SEM and considered significant for p < 0.05, *p < 0.05, **p < 0.01 ***p < 0.001.
Abbreviations
| PXE | = | pseudoxanthoma elasticum |
| MGP | = | matrix gla protein |
| OC | = | osteocalcin |
| MK4 | = | menaquinone-4 |
| MRP6 | = | multidrug resistance protein 6 |
| ABCC | = | ATP-binding cassette subfamily C |
| VKD | = | vitamin K-dependent proteins |
| GGCX | = | gamma-glutamyl carboxylase |
| VKORC1 | = | vitamin K 2,3 epoxide reductase |
| WT | = | wild-type |
Figures and Tables
Figure 1 Total calcium content in the muzzle skin of aging WT and Abcc6-/- mice. Calcium deposition in the Abcc6-/- mice was quantified by a colorimetric assay described in the Material and Methods section. Calcification initially increases with age with the significant increases occurring after 3 months of age. The largest increase in mineralization occurs between 3 weeks and 6 months. At around 12 months of age there is a gentle plateau in the rate of mineralization with no statistical increase measured throughout the rest of the ∼28–30 months life span of the mice.
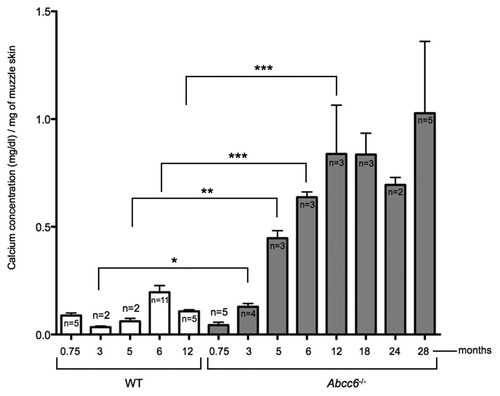
Figure 2 Calcification in the whole muzzle skin of mice fed diets enriched with 5 or 100 mg/kg of vitamin K1. No significant difference in calcium deposition was observed between the Abcc6-/- mice on normal or vitamin K1 enriched diets, with all Abcc6-/- mice having significantly more mineralization than WT controls. Mouse diets are described as ‘pre-onset’ of phenotype consist of mice fed on the diet from ∼day 21 for 16 weeks. ‘Post-onset’ corresponds to mice fed from ∼3 months of age (already possessing the calcification phenotype) for 12 weeks. In utero mouse samples were taken from mice which, when in utero, had mothers fed on the relevant diet and continued with the diet, through breast feeding and weaning up until 5 months of age.
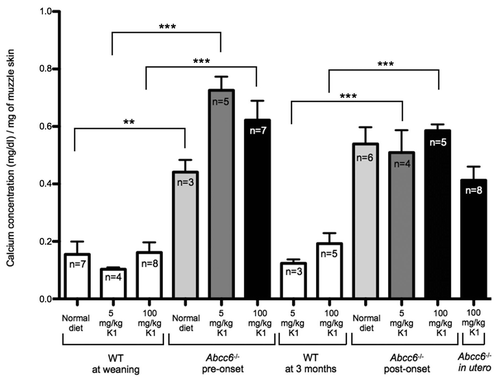
Figure 3 Muzzle skin calcification of mice fed diets enriched with 5 or 100 mg/kg of vitamin MK4. No significant difference in calcium deposition was observed between the Abcc6-/- mice on normal or vitamin MK4 enriched diets with all Abcc6-/- mice having significantly more mineralization than WT controls. Mouse diets are described as ‘pre-onset’ of phenotype consist of mice fed on the diet from ∼day 21 for 16 weeks. ‘Post-onset’ corresponds to mice fed from ∼3 months of age (already possessing the calcification phenotype) for 12 weeks. In utero mouse samples were taken from mice which, when in utero, had mothers fed on the relevant diet and continued with the diet, through breast feeding and weaning up until 5 months of age.
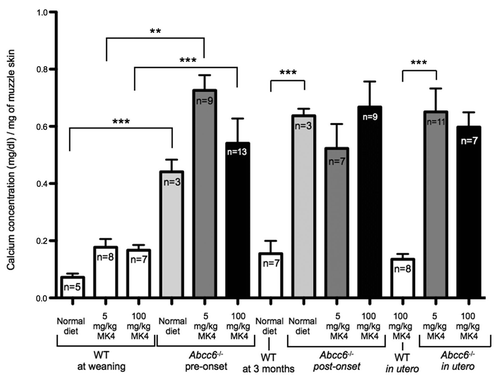
Figure 4 Histological evaluation of calcification in the vibrissae of WT and Abcc6-/- mice. Connective tissue calcium deposition was observed with Alizarin S Red staining in mice fed with either normal diet, or diet enriched in vitamin K1 or vitamin MK4 at 5 or 100 mg/kg. Sections of the muzzle skin were taken from each mouse from all mouse diet treatment groups. Mouse diets are described as ‘pre-onset’ of phenotype consist of mice fed with the diet from ∼day 21 for 16 weeks. ‘Post-onset’ corresponds to mice fed from ∼3 months of age (already possessing the calcification phenotype) for 12 weeks. In utero mouse samples were taken from mice which, when in utero, had mothers fed with the relevant diet and continued with the diet, through breast feeding and weaning up until 5 months of age. Size of hair follicle and depth of sections cut into the tissue were used as a guide to match relevant WT and Abcc6-/- sections. We observed no difference in placement or extent of calcium deposition. The same amount of staining occurred in the capsule surrounding the vibrissae in the whiskers in the Abcc6-/- mice fed control or either of the two vitamin K enriched diets.
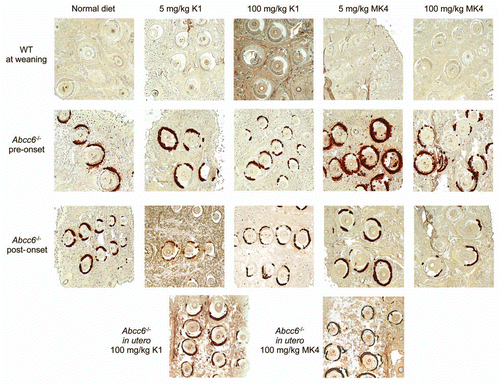
Figure 5 HPLC-MS analysis of serum levels of vitamin K1 from mice fed diets enriched with 5 or 100 mg/kg of vitamin K1. We measured a general, non-significant increase in circulating vitamin K1 levels in the serum of both WT and Abcc6-/- mice fed on a diet enriched with 5 mg/kg of vitamin K1. When fed with a diet enriched with 100 mg/kg vitamin K1, WT mice had significantly more circulating vitamin K1 than the Abcc6-/- mice, with no significant increase in circulating vitamin K1 measured in Abcc6-/- mice fed with this diet.
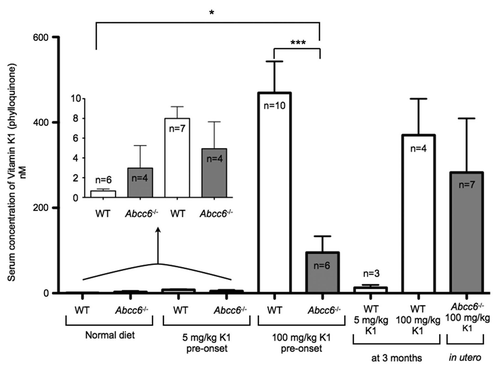
Figure 6 HPLC-MS analysis of serum levels of vitamin MK4 from mice fed diets enriched with 5 or 100 mg/kg of vitamin K1. When fed with a normal diet the serum taken from Abcc6-/- mice had significantly less circulating MK4 than WT controls. We measured a general, non-significant increase in circulating vitamin MK4 levels in the serum of WT mice fed on a diet enriched with 100 mg/kg of vitamin K1. WT mice fed with 100 mg/kg of vitamin K1 from pre-onset had significantly more circulating MK4 than Abcc6-/- mice. There was no increase in circulating MK4 levels in the serum of Abcc6-/- mice fed on any of the vitamin K1 diets.
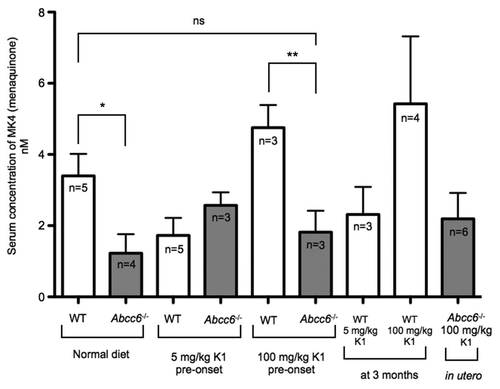
Figure 7 HPLC-MS analysis of serum levels of vitamin MK4 from mice fed diets enriched with 5 or 100 mg/kg of vitamin MK4. There was a significant increase in the levels of circulating MK4 in WT mice fed with a diet enriched with either a 5 mg/kg or 100 mg/mg of MK4. Additionally, we measured significant increases in circulating MK4 in Abcc6-/- mice fed with 100 mg/kg of MK4 in both pre- and post-onset groups. There was no significant increase in serum levels of MK4 in Abcc6-/- mice fed with the 5 mg/kg MK4 diet in either the pre- or post onset groups or in either of the in utero groups.
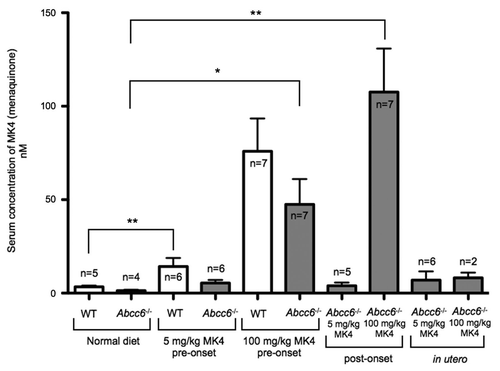
Figure 8 Immunofluorescent detection of MGP in the vibrissae's capsule of mice fed diets enriched with 100 mg/kg of vitamin K1 and MK4. Frozen serial sections from the muzzle tissue were used for both immunofluorescent and Alizarin S Red staining. We observed large positive staining of both ucMGP and tMGP (green) in the calcified regions of the vibrissae's capsule of Abcc6-/- mice fed with normal or vitamin K-enriched diets (Arrows), suggesting that the carboxylation status of MGP in these tissues is unaffected by increased dietary vitamin K1 or MK4. The counterstaining on immunofluorescent images was obtained with propidium iodide. The scale bar represents 200 µm.
Acknowledgments
The authors would like to acknowledge Dr. Jouni Uitto's laboratory for providing the Abcc6-/- mice used for this study. This work is supported by funding from NIH HL087289, AHA 11GRNT5840005 and a research grant from PXE France (O. Le Saux); Dr. A. Varadi was funded by NIH R01AR055225, by PXE Int. and Hungarian research grants OTKA CK 80135 and OTKA 81204.
References
- Bergen AA, Plomp AS, Schuurman EJ, Terry S, Breuning M, Dauwerse H, et al. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat Genet 2000; 25:228 - 231
- Le Saux O, Urban Z, Tschuch C, Csiszar K, Bacchelli B, Quaglino D, et al. Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat Genet 2000; 25:223 - 227
- Ringpfeil F, Lebwohl MG, Christiano AM, Uitto J. Pseudoxanthoma elasticum: Mutations in the MRP6 gene encoding a transmembrane ATP-binding cassette (ABC) transporter. Proc Natl Acad Sci USA 2000; 97:6001 - 6006
- Kool M, van der Linden M, de Haas M, Baas F, Borst P. Expression of human MRP6, a homologue of the multidrug resistance protein gene MRP1, in tissues and cancer cells. Cancer Res 1999; 59:175 - 182
- Beck K, Hayashi K, Nishiguchi B, Le Saux O, Hayashi M, Boyd CD. The distribution of Abcc6 in normal mouse tissues suggests multiple functions for this ABC transporter. J Histochem Cytochem 2003; 51:887 - 902
- Scheffer GL, Hu X, Pijnenborg AC, Wijnholds J, Bergen AA, Scheper RJ. MRP6 (ABCC6) detection in normal human tissues and tumors. Lab Invest 2002; 82:515 - 518
- Ilias A, Urban Z, Seidl TL, Le Saux O, Sinko E, Boyd CD, et al. Loss of ATP-dependent transport activity in pseudoxanthoma elasticum-associated mutants of human ABCC6 (MRP6). J Biol Chem 2002; 277:16860 - 16867
- Ringpfeil F, Pulkkinen L, Uitto J. Molecular genetics of pseudoxanthoma elasticum. Exp Dermatol 2001; 10:221 - 228
- Schinke T, McKee MD, Karsenty G. Extracellular matrix calcification: Where is the action?. Nat Genet 1999; 21:150 - 151
- Gheduzzi D, Boraldi F, Annovi G, DeVincenzi CP, Schurgers LJ, Vermeer C, et al. Matrix Gla protein is involved in elastic fiber calcification in the dermis of pseudoxanthoma elasticum patients. Lab Invest 2007; 87:998 - 1008
- Vermeer C. Gamma-carboxyglutamate-containing proteins and the vitamin K-dependent carboxylase. Biochem J 1990; 266:625 - 636
- Li Q, Jiang Q, Schurgers LJ, Uitto J. Pseudoxanthoma elasticum: Reduced gamma-glutamyl carboxylation of matrix gla protein in a mouse model (Abcc6-/-). Biochem Biophys Res Commun 2007; 364:208 - 213
- Borst P, van de Wetering K, Schlingemann R. Does the absence of ABCC6 (multidrug resistance protein 6) in patients with Pseudoxanthoma elasticum prevent the liver from providing sufficient vitamin K to the periphery?. Cell Cycle 2008; 7:1575 - 1579
- Vanakker OM, Martin L, Gheduzzi D, Leroy BP, Loeys BL, Guerci VI, et al. Pseudoxanthoma elasticum-like phenotype with cutis laxa and multiple coagulation factor deficiency represents a separate genetic entity. J Invest Dermatol 2007; 127:581 - 587
- Li Q, Uitto J. The mineralization phenotype in Abcc6 (-/-) mice is affected by Ggcx gene deficiency and genetic background—a model for pseudoxanthoma elasticum. J Mol Med 88:173 - 181
- Vanakker OM, Martin L, Schurgers LJ, Quaglino D, Costrop L, Vermeer C, et al. Low serum vitamin K in PXE results in defective carboxylation of mineralization inhibitors similar to the GGCX mutations in the PXE-like syndrome. Lab Invest 2010; 90:895 - 905
- Shearer MJ. Vitamin K. Lancet 1995; 345:229 - 234
- Okano T, Shimomura Y, Yamane M, Suhara Y, Kamao M, Sugiura M, et al. Conversion of phylloquinone (Vitamin K1) into menaquinone-4 (Vitamin K2) in mice: Two possible routes for menaquinone-4 accumulation in cerebra of mice. J Biol Chem 2008; 283:11270 - 11279
- Hara K, Akiyama Y, Nakamura T, Murota S, Morita I. The inhibitory effect of vitamin K2 (menatetrenone) on bone resorption may be related to its side chain. Bone 1995; 16:179 - 184
- Schurgers LJ, Teunissen KJ, Hamulyak K, Knapen MH, Vik H, Vermeer C. Vitamin K-containing dietary supplements: Comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood 2007; 109:3279 - 3283
- Will BH, Suttie JW. Comparative metabolism of phylloquinone and menaquinone-9 in rat liver. J Nutr 1992; 122:953 - 958
- Schurgers LJ, Spronk HM, Soute BA, Schiffers PM, DeMey JG, Vermeer C. Regression of warfarin-induced medial elastocalcinosis by high intake of vitamin K in rats. Blood 2007; 109:2823 - 2831
- Klement JF, Matsuzaki Y, Jiang QJ, Terlizzi J, Choi HY, Fujimoto N, et al. Targeted ablation of the abcc6 gene results in ectopic mineralization of connective tissues. Mol Cell Biol 2005; 25:8299 - 8310
- Gorgels TG, Hu X, Scheffer GL, van der Wal AC, Toonstra J, de Jong PT, et al. Disruption of Abcc6 in the mouse: Novel insight in the pathogenesis of pseudoxanthoma elasticum. Hum Mol Genet 2005; 14:1763 - 1773
- Jiang Q, Li Q, Uitto J. Aberrant mineralization of connective tissues in a mouse model of pseudoxanthoma elasticum: Systemic and local regulatory factors. J Invest Dermatol 2007; 127:1392 - 1402
- Thijssen HH, Drittij-Reijnders MJ, Fischer MA. Phylloquinone and menaquinone-4 distribution in rats: Synthesis rather than uptake determines menaquinone-4 organ concentrations. J Nutr 1996; 126:537 - 543
- Le Saux O, Martin L. [The molecular genetics of pseudoxanthoma elasticum]. Ann Dermatol Venereol 2001; 128:943 - 946
- Chassaing N, Martin L, Calvas P, Le Bert M, Hovnanian A. Pseudoxanthoma elasticum: A clinical, pathophysiological and genetic update including 11 novel ABCC6 mutations. J Med Genet 2005; 42:881 - 892
- Pfendner EG, Vanakker OM, Terry SF, Vourthis S, McAndrew PE, McClain MR, et al. Mutation detection in the ABCC6 gene and genotype-phenotype analysis in a large international case series affected by pseudox-anthoma elasticum. J Med Genet 2007; 44:621 - 628
- Uitto J, Pulkkinen L, Ringpfeil F. Molecular genetics of pseudoxanthoma elasticum: A metabolic disorder at the environment-genome interface?. Mol Med Today 2001; 7:13 - 17
- Shearer MJ. Role of vitamin K and Gla proteins in the pathophysiology of osteoporosis and vascular calcification. Curr Opin Clin Nutr Metab Care 2000; 3:433 - 438
- Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MH, van der Meer IM, et al. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: The Rotterdam study. J Nutr 2004; 134:3100 - 3105
- Matsuzaki Y, Nakano A, Jiang QJ, Pulkkinen L, Uitto J. Tissue-specific expression of the ABCC6 gene. J Invest Dermatol 2005; 125:900 - 905
- Bolisetty S, Gupta JM, Graham GG, Salonikas C, Naidoo D. Vitamin K in preterm breastmilk with maternal supplementation. Acta Paediatr 1998; 87:960 - 962
- McGee-Russell SM. Histochemical methods for calcium. Journal of Histochemistry & Cytochemistry 1958; 6:22 - 42
- Spronk HM, Soute BA, Schurgers LJ, Thijssen HH, De Mey JG, Vermeer C. Tissue-specific utilization of menaquinone-4 results in the prevention of arterial calcification in warfarin-treated rats. J Vasc Res 2003; 40:531 - 537