Abstract
Mesenchymal stem/stromal cells (MSCs) are fibroblastoid cells capable of long-term expansion and skeletogenic differentiation. While MSCs are known to originate from neural crest and mesoderm, immediate mesodermal precursors that give rise to MSCs have not been characterized. Recently, using human embryonic stem cells (hESCs), we demonstrated that mesodermal MSCs arise from APLNR+ precursors with angiogenic potential, mesenchymoangioblasts, which can be identified by FGF2-dependent colony-forming assay in serum-free semisolid medium. In this overview we provide additional insights on cellular pathways leading to MSC establishment from mesoderm, with special emphasis on endothelial-mesenchymal transition as a critical step in MSC formation. In addition, we highlight an essential role of FGF2 in induction of angiogenic cells with potential to transform into MSCs (mesenchymoangioblasts) or hematopoietic cells (hemangioblasts) from mesoderm, and discuss correlations of our in vitro findings with the course of angioblast development during embryogenesis.
Mesenchymal stem/stromal cells (MSCs) are defined as multipotent fibroblastoid cells that give rise to cells of the skeletal connective tissue including osteoblasts, chondrocytes and adipocytes.Citation1–Citation4 Although MSCs were described more than 40 years ago and are widely used for cellular therapies, very little knowledge exists regarding the developmental origins of MSCs in the embryo, the hierarchy of MSC progenitors or heterogeneity of MSCs within tissues. It has been demonstrated that during embryonic development, MSCs arise from a two major sources: neural crest and mesoderm.Citation5–Citation7 Using Cre-recombinase lineage tracing experiments, Takashima et al. identified Sox1+ neuroepithelium as pre-cursors of MSCs of neural crest origin. However, direct precursors of mesoderm-derived MSCs were unknown. To identify these precursors, we employed human embryonic stem cells (hESCs) directed toward mesendodermal differentiation in coculture with mouse bone marrow stromal cells OP9,Citation8 using the experimental approach depicted in . As shown in this differentiation system, mesoderm reminiscent of lateral plate/extraembryonic mesoderm in the embryo can be identified by expression of apelin receptor (APLNR), otherwise known as angiotensin receptor like-1 receptor. Because we observed a positive selective effect of FGF2 on production of mesenchymal cells from hESCs in OP9 coculture, we decided to test whether FGF2 can induce the formation of colonies with mesenchymal potential from APLNR+ mesodermal cells. Indeed, when we isolated APLNR+ cells from hESCs differentiated on OP9 for 2 days and placed them in serum-free semisolid medium containing FGF2, we observed the formation of sharply-circumscribed spheroid colonies formed by tightly packed cells with a gene expression profile representative of embryonic mesenchyme originating from lateral plate/extraembryonic mesoderm and CD140a+CD146+C D90+CD56+CD166+CD31−CD43−CD45− phenotype typical of mesenchymal cells. Based on cellular composition, we designated these colonies as mesenchymal (MS) colonies and cells forming these colonies as MS colony-forming cells (MS-CFCs). MS colony formation required serum-free medium and was solely dependent on FGF2 as a colony-forming factor. MS colonies were significantly enhanced by PDGF-BB, but suppressed by VEGF, TGFβ1 and Activin A. When transferred to the adherent cultures in serum-free medium with FGF2, individual MS colonies gave rise to multi-potential mesenchymal cell lines with typical phenotype (CD146+ CD105+ CD73+ CD31− CD43/45−), differentiation (chondro-, osteo- and adipogenesis) and robust proliferation (>80 doublings) potentials. Using single cell deposition assay, chimeric hESC lines and time-lapse studies we demonstrated the clonality/single cell origin of MS colonies.
MS-CFCs could be detected only transiently, with a major peak on day 2 of hESC differentiation and disappeared after 4 days of differentiation. Notably, MS-CFC activity was developed prior to the expression of CD73 and CD105 MSC markers and upregulation of MSC-related genes, i.e., before onset of mesenchymogenesis. APLNR+ cells isolated from hESC cultures differentiated for 3 days also formed colonies in response to FGF2; however, the vast majority of these colonies were composed of blood cells and had a morphology similar to the previously described blast (BL) or hemangioblast colonies, which identify a common precursor for hematopoietic and endothelial cells.Citation9,Citation10
To fully evaluate the differentiation potential of MS colonies, we collected these colonies from semisolid cultures and placed them back on OP9 feeders, which are known to support development of a broad range of mesodermal lineage cells, including hematopoietic, vascular and cardiac.Citation11–Citation13 Using this approach, we confirmed that individual BL colonies possess hemangioblastic potential, i.e., generate both hematopoietic and endothelial cells. When MS colonies were picked from clonogenic cultures and cultured on OP9, we found that the majority of cells differentiated into CD146+CD31−CD43/CD45− mesenchymal cells as expected. However, we also discovered that MS colonies gave rise to CD31/VE-cadherin+CD43/45− endothelial cells, indicating that MS colonies similar to BL colonies possess endothelial potential. The endothelial potential of MS colonies was also confirmed by demonstration of tube formation by MS colonies grown on Matrigel. In contrast, MSC lines derived from MS colonies did not produce any endothelial cells after coculture with OP9 indicating a progressive restriction of differentiation potential following MSC formation. Because single MS-CFC shows potential to form endothelium and MSCs, we designated the MSC precursor identified by this colony-forming assay as mesenchymoangioblast.
To define more precisely the cellular events leading to establishing MSCs, we examined the formation of MS colonies using time-lapse cinematography and analyzed the kinetic of their angiogenic potential. As demonstrated by time-lapse studies, APLNR+ mesodermal cells placed in semisolid medium possessed a high motility, which was more pronounced before and during the first cell division. Following several divisions, single APLNR+ cells formed a core, an immotile structure composed of a small number of tightly packed cells. While APLNR+ mesodermal cells lacked endothelial gene expression, molecular profiling of MS colonies at the core stage revealed that these cells acquired angioblastic gene expression profile as indicated by upregulation of FLT1, TEK, CDH5 (VE-cadherin), PECAM1 (CD31), FLI1, SELE (ELAM-1) and ICAM2 endothelial genes. When we collected MS cores (day 3 of clonogenic culture) and placed them on OP9, they formed predominantly VE-cadherin+ endothelial clusters, strongly indicating the endothelial nature of the core-forming cells. Subsequently, cells at the periphery of the core underwent endothelial-mesenchymal transition (EndMT) and formed a shell of tightly packed spindle-like cells around the core. When we picked colonies at this stage (day 6 of colony-forming culture) and placed them on OP9, most of the colonies (>70%) grew cell clusters composed of endothelial and mesenchymal cells. In contrast, mature MS colonies collected on day 12 of clonogenic culture formed predominantly clusters of mesenchymal cells, indicating a progressive loss of endothelial potential following colony maturation. Although no CD31 expression was detected in the mesenchymal cells composing mature MS colonies, these cells retained several endothelial traits including surface expression of endothelial tyrosine kinase (TEK or TIE2), FLT1 (VEGFR1) and endomucin. The critical role of EndMT in MS colony formation and MSC development was also congruous with our observation of the suppressive effect of VEGF, a known inhibitor of EndMT,Citation14,Citation15 on MS colonies. When VEGF was added to MS clonogenic cultures, hESC-derived mesodermal cells were capable of forming angiogenic cores; however, these cores did not transform into mesenchymal cells, indicating that VEGF abrogates MS colony development at the core stage through inhibition of EndoMT. The schematic diagram demonstrating development of mesodermal MSCs is presented in .
The close relationship between endothelial and hematopoietic cell development was recognized more than 130 years ago (reviewed by ref. Citation16) and confirmed in multiple modern studies.Citation9,Citation17–Citation22 However, the association between endothelial pre-cursors and MSCs during development was not well established, although cells with endothelial and mural cell potential were identifiedCitation23 and the critical role of EndMT in the formation of endocardial cushionCitation24 and testicular cordsCitation25 in the embryo was acknowledged. Our hESC-based in vitro studies indicated that formation of mesodermal MSCs proceed through the endothelial stage and likely included at least two successive cycles of cell transitions. Initially APLNR+ mesoderm, which consists of fibroblast-like migratory cells, give rise to core structures composed of tightly packed endothelial cells in response to FGF2. Subsequently, endothelial cells forming cores undergo epithelial-mesenchymal transition, i.e., EndMT and form MSCs. The question remains how well this in vitro model reflects in vivo development. Although only sparse data exist regarding MSC precursors in the embryo, development of angiogenic hematopoietic precursors, hemangioblasts was studied more extensively in mammals and birds, and therefore parallels between in vivo and in vitro studies can be drawn. As we demonstrated,Citation8 APLNR+ mesodermal cells collected from hESCs differentiated on OP9 for 3 days formed disperse BL colonies that identify hemangioblasts in vivo and in vitro.Citation9,Citation26 Similar to MS colonies, the development of BL colonies required FGF2 and proceeded through angiogenic core formation. However, in contrast to MS cores, BL cores transformed into blood cells, i.e., underwent endothelial-hematopoietic transformation (see ). Importantly, in vivo studies identified FGF2 as the essential factor in hemangioblast inductionCitation27 analogous to our in vitro observation. In chicken embryo, the activation of FGF signaling leads to aggregation of migrating mesodermal cells adjacent to the endoderm, upregulation of VEGFR2 (KDR) expression, and subsequent formation of angioblasts and hemangioblasts.Citation28–Citation30 This sequence of events leading to hemangioblast development in vivo considerably resembles what we observed in vitro, and highly suggests accurate recapitulation of embryonic development by our hESC differentiation model. Therefore, searching for an in vivo equivalent of mesenchymonagioblast would be a reasonable next step.
In addition to embryonic development, EndMT is also implicated in several pathologies including cancer progression and development of cardiac and renal fibrosis.Citation31–Citation34 Recently, Olsen group revealed that endothelial cells could be transformed directly into MSCs through overexpression of ALK2 or its activation by TGFβ2 or BMP4,Citation15 indicating that endothelial cells could be an important source of MSCs in postnatal life. Conversely, the transition from MSCs to endothelial cells, has been also described in reference Citation35. Based on these observations, a cycle of cell-fate transition from endothelium to MSCs and back to endothelium was proposed as a circuit controlling stem cell state.Citation36 Since multiple parallels could be drawn between EndMT described in adult tissues and during hESC differentiation, one may wonder whether bipotential cells with endothelial and MSC potential similar to embryonic mesenchymoangioblasts are present and constitute an important element of EndMT circuit in adults.
In conclusion, the identification of mesenchymoangioblast as a clonogenic precursor of mesoderm-derived MSCs is an important step toward defining pathways of MSC development and specification. In addition, the demonstration of MSC formation from mesoderm through EndMT provides new insights into the mechanisms involved in establishment of MSCs.
Figures and Tables
Figure 1 Schematic diagram of the experimental approach used to identify precursors and cellular events leading to formation of mesoderm-derived MSCs. hESCs were committed to mesendodermal differentiation through coculture with OP9 for 2 days. APLNR+ mesodermal cells were selected using magnetic sorting. In serum-free semisolid medium, APLNR+ cells grew into FGF2-dependent compact spheroid colonies composed of mesenchymal cells. MS colonies were formed through establishment of tightly-packed single cell-derived cores (day 3 of clonogenic culture), which expanded into spheroid colonies (days 6 and 12 of clonogenic culture). To evaluate differentiation potential, MS colonies were collected at different stages of clonogenic culture and placed on OP9. The presence of endothelial and mesenchymal cells after coculture of MS colonies with OP9 was evaluated by flow cytometry and immunofluorescence. In addition, colonies at core stage (day 3 of clonogenic culture) and mature colonies (day 12 of clonogenic cultures) were collected for molecular profiling studies. To generate clonal MSC lines, individual mature colonies were plated on the collagen/fibronectin-coated plastic and cultured in presence of FGF2.
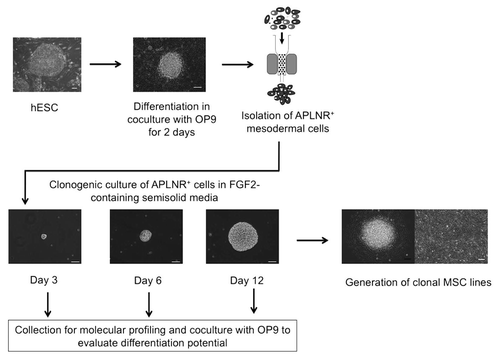
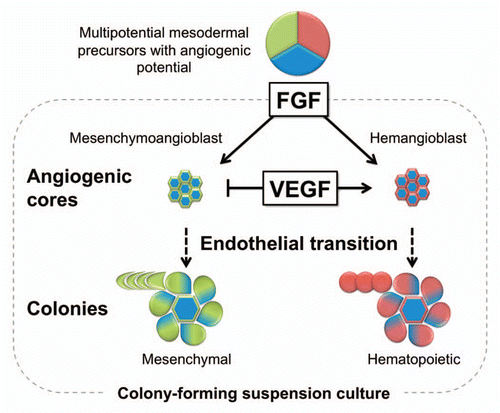
Figure 2 A model of mesoderm-derived MSC development from hESCs. Coculture with OP9 stromal cells predominantly induces hESC differentiation toward APLNR+ mesoderm. APLNR+ population contains angiogenic mesodermal precursors with either mesenchymal (mesenchymoangioblast) or hematopoietic (hemangioblast) potentials. Mesenchymoangioblasts and hemangioblasts arise sequentially during differentiation and can be revealed by MS and BL colony formation in response to FGF2. Development of MS and BL colonies in semisolid media proceed through a core stage at which APLNR+ cells form clusters of tightly packed cells with angiogenic potential. Subsequently, core-forming cells undergo EndMT giving rise to mesenchymal cells, which form a shell around the core developing into a mature MS colony. VEGF, EndMT inhibitor, blocks MS colony-formation at core stage. The ability of MS-CFCs to generate mesenchymal and endothelial cells can be revealed by coculture of individual colonies with OP9. Similar to MS colonies, BL colonies are formed through establishment of angiogenic core. However, hemangioblast core-forming cells undergo endothelial-hematopoietic transition and grew hematopoietic cells around the core.
References
- Delorme B, Chateauvieux S, Charbord P. The concept of mesenchymal stem cells. Regen Med 2006; 1:497 - 509
- Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts and assays. Cell Stem Cell 2008; 2:313 - 319
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997; 276:71 - 74
- Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 1974; 17:331 - 340
- Morikawa S, Mabuchi Y, Niibe K, Suzuki S, Nagoshi N, Sunabori T, et al. Development of mesenchymal stem cells partially originate from the neural crest. Biochem Biophys Res Commun 2009; 379:1114 - 1119
- Takashima Y, Era T, Nakao K, Kondo S, Kasuga M, Smith AG, et al. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell 2007; 129:1377 - 1388
- Olsen BR, Reginato AM, Wang W. Bone development. Annu Rev Cell Dev Biol 2000; 16:191 - 220
- Vodyanik MA, Yu J, Zhang X, Tian S, Stewart R, Thomson JA, et al. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell 2010; 7:718 - 729
- Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development 1998; 125:725 - 732
- Kennedy M, D'Souza SL, Lynch-Kattman M, Schwantz S, Keller G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood 2007; 109:2679 - 2687
- Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science 1994; 265:1098 - 1101
- Vodyanik MA, Bork JA, Thomson JA, Slukvin II. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood 2005; 105:617 - 626
- Schroeder T, Fraser ST, Ogawa M, Nishikawa S, Oka C, Bornkamm GW, et al. Recombination signal sequence-binding protein Jkappa alters mesodermal cell fate decisions by suppressing cardiomyogenesis. Proc Natl Acad Sci USA 2003; 100:4018 - 4023
- Paruchuri S, Yang JH, Aikawa E, Melero-Martin JM, Khan ZA, Loukogeorgakis S, et al. Human pulmonary valve progenitor cells exhibit endothelial/mesenchymal plasticity in response to vascular endothelial growth factor-A and transforming growth factor-beta2. Circ Res 2006; 99:861 - 869
- Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med 2010; 16:1400 - 1406
- Maximow AA. Relation of blood cells to connective tissues and endothelium. Physiological Reviews 1924; 4:533 - 563
- Nishikawa SI, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development 1998; 125:1747 - 1757
- Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell 2008; 3:625 - 636
- Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 2010; 464:112 - 115
- Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 2010; 464:116 - 120
- Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 2010; 464:108 - 111
- Taoudi S, Medvinsky A. Functional identification of the hematopoietic stem cell niche in the ventral domain of the embryonic dorsal aorta. Proc Natl Acad Sci USA 2007; 104:9399 - 9403
- Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 2000; 408:92 - 96
- Nakajima Y, Yamagishi T, Hokari S, Nakamura H. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-beta and bone morphogenetic protein (BMP). Anat Rec 2000; 258:119 - 127
- Combes AN, Wilhelm D, Davidson T, Dejana E, Harley V, Sinclair A, et al. Endothelial cell migration directs testis cord formation. Dev Biol 2009; 326:112 - 120
- Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature 2004; 432:625 - 630
- Flamme I, Risau W. Induction of vasculogenesis and hematopoiesis in vitro. Development 1992; 116:435 - 439
- Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol 1995; 11:73 - 91
- Cox CM, Poole TJ. Angioblast differentiation is influenced by the local environment: FGF-2 induces angioblasts and patterns vessel formation in the quail embryo. Dev Dyn 2000; 218:371 - 382
- Flamme I, Breier G, Risau W. Vascular endothelial growth factor (VEGF) and VEGF receptor 2 (flk-1) are expressed during vasculogenesis and vascular differentiation in the quail embryo. Dev Biol 1995; 169:699 - 712
- Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol 2008; 19:2282 - 2287
- Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. Br J Cancer 2008; 99:1375 - 1379
- Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res 2007; 67:10123 - 10128
- Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 2007; 13:952 - 961
- Conrad C, Niess H, Huss R, Huber S, von Luettichau I, Nelson PJ, et al. Multipotent mesenchymal stem cells acquire a lymphendothelial phenotype and enhance lymphatic regeneration in vivo. Circulation 2009; 119:281 - 289
- Shoshani O, Zipori D. Transition of endothelium to cartilage and bone. Cell Stem Cell 2011; 8:10 - 11