Abstract
The red-eared slider turtle (Trachemys scripta elegans) has a well-developed natural tolerance for oxygen deprivation that derives from biochemical adaptations, including anoxia-induced suppression of metabolic rate. We hypothesized that mechanisms that suppress ATP-expensive cell cycle activity would contribute significantly to establishing the hypometabolic state during anaerobiosis. Cyclin D1 is a critical regulator of the G1 phase of the cell cycle and is regarded as key to initiating cell proliferation. The relative protein expression of cyclin D1 was analyzed in both whole-cell and nuclear fractions of liver, kidney and skeletal muscle from turtles exposed to 5 or 20 h of submergence anoxia. Expression of cyclin D1 in both total and nuclear fractions decreased significantly under anoxia in liver and kidney as compared with aerobic controls, but no significant change occurred in muscle. The relative phosphorylation state of cyclin D1 (threonine 286) was also unchanged during anoxia in all tissues. Since phosphorylation of threonine 286 is necessary for proteasomal degradation of cyclin D1, this implies that an alternative mechanism is responsible for cyclin D1 suppression in anoxia. Levels of cyclin D1 mRNA transcripts did not change under anoxia in any tissue, so a post-transcriptional method of regulation was implicated. Analysis of the 3’UTR of cyclin D1 showed the presence of both an AU-rich region and a conserved binding site for microRNA-16-1 and microRNA-15a. Levels of both microRNAs increased in liver and kidney (but not in muscle) under anoxic conditions, implicating microRNA inhibition of mRNA translation as the mechanism underlying the suppression of cyclin D1 protein levels in the anoxic turtle.
Keywords: :
Introduction
The red-eared slider (Trachemys scripta elegans) is a freshwater turtle that can survive for extended periods of time without breathing oxygen. This supports long dives necessary for feeding or predator avoidance, as well as weeks or months of winter hibernation at the bottom of ponds and rivers.Citation1 Some turtle species can take up significant amounts of oxygen from water by nonpulmonary means. However, T. s. elegans is poor at this, and furthermore, if turtles overwinter in ice-covered ponds, oxygen can be quickly depleted by the respiration of other organisms, resulting in hypoxic or anoxic water.Citation1 Oxygen deprivation has severe consequences for cellular energy production, and for intolerant species, even small fluctuation in oxygen availability can be lethal.Citation2 Turtles are able to achieve this impressive facultative anaerobiosis using mechanisms including: (1) strong metabolic rate suppression, (2) a high capacity for glycolytic energy production and (3) effective methods for dealing with end products (acid buffering, lactate storage in shell).Citation3,Citation4
Molecular mechanisms of metabolic rate depression include a global suppression of energy-utilizing processes and a reprioritization of ATP use to support cell functions that are vital for survival.Citation5 Cell functions that are strongly suppressed include protein synthesis and degradation, gene transcription and ATP-dependent ion pumps.Citation6 However, selected cell functions are relatively enhanced under anoxic conditions, especially those that preserve and stabilize cellular macromolecules (such as antioxidant defenses and chaperone proteins).Citation7,Citation8 Entry into a hypometabolic, energy-restricted state is not the time for a major reorganization of cellular metabolism and, therefore, does not involve extensive changes in gene and protein expression. Instead, the types of regulatory mechanisms that are best suited for anoxia survival are those that are broadly applicable to multiple metabolic processes, readily coordinated by extracellular stimuli, easily induced and readily reversed.Citation3 Reversible phosphorylation of proteins has proven to be one such regulatory mechanism that exerts wide-ranging control over metabolism under anoxia.Citation1-Citation3 Interestingly, the characteristics of microRNA regulation toward mRNA transcripts also fulfills these requirements and may, therefore, be key to both translational suppression and transcript storage in the anoxic state.
MicroRNAs (miRNAs) are short, non-coding RNAs capable of regulating protein expression. These 18–25 nucleotide transcripts bind with full or partial complementarity to mRNA targets, resulting in either translational inhibition or degradation of that target.Citation9-Citation11 A single miRNA may target multiple mRNAs, and a single mRNA may have multiple miRNA binding sites.Citation12,Citation13 The degree and extent of microRNA binding creates a complex regulatory system that is predicted to be involved in almost every aspect of biological function. To date, microRNAs are known to be critically involved in development, cell differentiation, apoptosis, cell cycle control, stress response and disease pathogenesis.Citation12,Citation14-Citation16 As such, regulation of translation by microRNA action could also be an important regulatory mechanism in controlling metabolic rate. Indeed, significant changes in microRNA expression have already been identified in other models of metabolic rate depression, including freeze tolerance in wood frogsCitation17 and hibernation in squirrelsCitation18 and bats.Citation19 Their involvement in anoxia survival of turtles is explored in this paper.
Given the high energetic cost of cell proliferation, it would make sense that cell division is suppressed to a minimum under oxygen-limiting conditions. Previous reports have suggested that a withdrawal of ATP supply induces proliferation arrest in the G1 phase of the cell cycle.Citation20 Indeed, a recent study of freeze-tolerant and anoxia-tolerant wood frogs, Rana sylvatica, showed that anoxia exposure induced a significant decrease in critical cell cycle components (type D1 and E1 cyclins, type 2, 4 and 6 cyclin-dependent kinases) as well as an increase in cell cycle inhibitors (p16INK4A and p27KIP1) that are specific to G1/G0 arrest.Citation21 Similar mechanisms of G1/G0 cell cycle arrest have been proposed for T. s. elegans during anaerobiosis.Citation22
The cell cycle is incredibly complex, involving many different proteins and protein complexes at each phase of the cycle.Citation23 However, a critical regulatory point is the initiation phase (G1), an arrest checkpoint shown to be involved in multiple models of anoxia tolerance and hypometabolism.Citation24-Citation26 Activities of the cyclin/Cdk complexes, critical to cell cycle control, are dictated by the availability of the associated cyclins. Whereas Cdks are constitutively expressed throughout the cell cycle, cyclins are translated and shuttled to the nucleus immediately before they are required.Citation27 Importantly, cyclin D1 is specific to the G1 phase of the cell cycle, associating only with Cdk 4 or 6 and promoting cell cycle initiation.Citation28 Unlike other cyclins, expression of cyclin D1 is strongly dependent on extracellular mitogenic cues and is regarded as a critical component in initiating cellular proliferation and regulating the G1 phase of the cell cycle.Citation26,Citation28,Citation29 Cyclin D1 abundance is influenced by a combination of transcriptional and translational rates as well as protein stability.Citation27,Citation30
Many studies have reported differential regulation of cyclin D1 and its effects on the proliferative state of diseased cells.Citation31-Citation33 Mutations in the 3’UTR of cyclin D1 are also responsible for the hyperproliferative state associated with a variety of tumors types.Citation34 Cells expressing a cyclin D1 that lacks a 3’UTR also show a higher rate of proliferation.Citation34,Citation35 This evidence suggests the presence of negative regulatory elements in the 3’UTR of cyclin D1. It has been suggested that AU-rich elements (AREs) located within the 3’UTR are responsible for cyclin D1 destabilization, with the loss of AREs leading to an overexpression of cyclin D1 protein and a hyperproliferative phenotype.Citation36 Other studies have discovered that members of the microRNA-15/16 family can also target the 3’UTR of cyclin D1 mRNA, providing a form of post-transcriptional regulation.Citation35,Citation37-Citation39 Importantly, these studies have shown that the deletion of the cyclin D1 3’UTR leads to the loss of both AREs and microRNA binding sites, resulting in an increase in cyclin D1 translation and overexpression.Citation35 Taken together, these studies suggest that the 3’UTR of cyclin D1 may be critical to cyclin D1 regulation and, as a result, may be important in regulating cellular proliferation during periods of cellular stress.
To examine how the cell cycle is regulated in response to anoxia in the red-eared slider turtle, this study examines the comparative levels of mRNA and protein (total and phosphorylated) of the cell cycle initiator, cyclin D1. Post-transcriptional regulation of cyclin D1 expression is also assessed, particularly the role of microRNA-16-1 and microRNA-15a in regulating cyclin D1. The analysis yields a picture of the control of cyclin D1 in turtle tissues and its adaptive regulation in anoxia-induced metabolic rate depression.
Results
Cyclin D1 expression.
Expression levels of cyclin D1 protein decreased significantly in response to anoxia exposure in liver and kidney of turtles but did not change skeletal muscle (). In liver, cyclin D1 protein decreased to 59 ± 2% of the aerobic control value after 5 h anoxia and remained low at 62 ± 9% of control after 20 h anoxia (p < 0.05). A similar response occurred in kidney, where cyclin D1 protein decreased to 67 ± 8% of the control value in response to 20 h of anoxic submergence (p < 0.05).
Figure 1. Effect of anoxia exposure on the relative protein expression of (A) cyclin D1, (B) cyclin D1 localized to the nucleus, and (C) phosphorylated cyclin D1 (Thr286) in liver, kidney and skeletal muscle from aerobic control and anoxic (5 and 20 h) turtles. Representative bands show immunoblot intensity for control and experimental groups. Histograms show normalized expression levels for control vs. 5 and 20 h anoxic conditions; data are means ± SEM (n = 3–4 independent trials on tissue from different animals). * Indicates a significant difference from the corresponding control (p < 0.05).
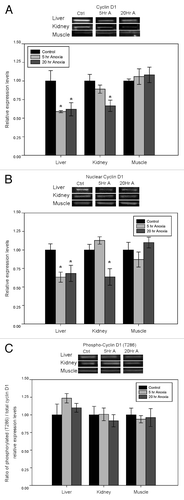
Cyclin D1 expression was also evaluated in the nuclear compartment of turtle tissues and showed a similar pattern to that described above for total protein extracts (). Nuclear levels of cyclin D1 protein decreased significantly in liver to 63 ± 7% after 5 h anoxia and 68 ± 11% after 20 h anoxia as compared with aerobic control values (p < 0.05). Nuclear expression of cyclin D1 also decreased in kidney to 67 ± 8% of the control value after 20 h anoxia (p < 0.05). No significant changes in nuclear cyclin D1 content were seen in skeletal muscle during anoxia.
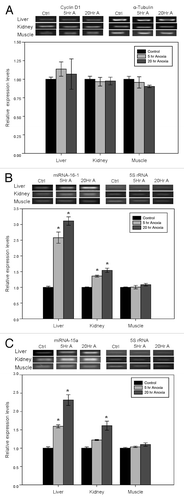
Several studies have reported that the cyclin D1 degradation is dependent on the initial phosphorylation of the threonine 286 residue, followed by poly-ubiquitination and subsequent proteasomal degradation. To assess the state of cyclin D1 degradation, we analyzed relative phosphorylation levels at T286 using antibodies specific for the phosphorylated peptide and then calculated the ratio of phosphorylated cyclin D1 (T286) expression to total cyclin D1 protein in tissues from control vs. anoxic turtles. This ratio did not change significantly between control and anoxic states in any of the three tissues (). Expression levels of cyclin D1 mRNA were also analyzed using RT-PCR. Unlike the stress response seen for cyclin D1 protein in liver and kidney, no significant changes in cyclin D1 transcript levels were detected in any tissue ().
Figure 2. Effect of anoxia exposure on the relative expression of (A) cyclin D1 mRNA, (B) microRNA-16–1 and (C) microRNA-15a in liver, kidney and skeletal muscle from aerobic control and anoxic (5 and 20 h) turtles. Representative bands show RNA transcript levels amplified by RT-PCR. Band intensities from the RT-PCR samples were normalized to either α-tubulin (cyclin D1) or 5S rRNA (microRNA) bands amplified from the same sample. Histograms show normalized expression levels for control vs. 5 and 20 h anoxic conditions; data are means ± SEM (n = 3–4 independent trials on tissue from different animals). * Indicates a significant difference from the corresponding control (p < 0.05).
Identification of regulatory elements within the cyclin D1
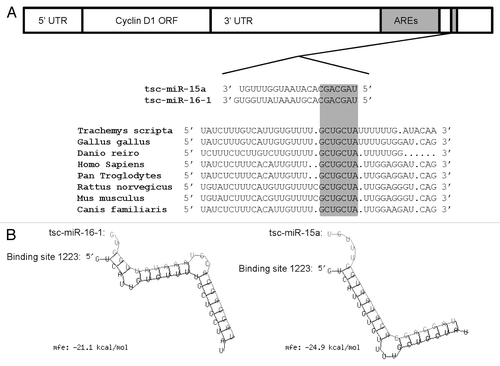
3’ UTR. The 3’UTR sequence of cyclin D1 mRNA from T. s. elegans was obtained using 3’ RACE and analyzed for elements that could potentially regulate cyclin D1 translation. We found that the 3’UTR contained a cluster of four AU elements (AUUUA) within a ~275 base region (of the 2,300 bp sequenced) (GenBank# JQ670888). An analysis of putative microRNA binding sites was then performed using both TargetScan 5.2 and RNAhybrid. Results showed that the 3’UTR of turtle cyclin D1 contained binding sequences for both microRNA-16-1 and microRNA-15a starting 1,223 bp within the 3’UTR sequence. Importantly, one binding site shared by both microRNA-16-1 and microRNA-15a, starting 1,223 bp within the 3’ UTR, was identified and found to be conserved in multiple vertebrate groups, including fish (Danio reiro), reptiles (T. s. elegans), birds (Gallus gallus), and mammals (Homo sapiens, Pan troglodytes, Rattus norvegicus, Mus musculus, Canis familiaris) ().
Figure 3. Theoretical binding of microRNA-16–1 and microRNA-15a to a conserved region in the 3′ UTR of the turtle cyclin D1 gene. (A) Conservation analysis of the microRNA-16–1/-15a binding site in the cyclin D1 gene from the red-eared slider turtle (Trachemys scripta elegans), chicken (Gallus gallus), zebra fish (Danio rerio), human (Homo sapiens), common chimpanzee (Pan troglodytes), brown rat (Rattus norvegicus), house mouse (Mus musculus) and domestic dog (Canis familiaris).The seed region sequence (shaded) shows 100% conservation between the eight sequences. (B) Predicted binding structures of both tsc-miR-16–1 and tsc-miR-15a when binding to the 3′UTR of T. s. elegans cyclin D1, as determined from the RNAhybrid program.
Minimum free energy of cyclin D1:microRNA binding.
To determine the likelihood of cyclin D1:microRNA binding, we investigated the theoretical minimum free energy (mfe) of binding (kcal/mol) of the RNA duplex formation. The theoretical mfe was calculated using the program RNAhybrid. Theoretical predictions of RNA duplex formation and thermodynamic binding parameters for microRNA-16-1 and microRNA-15a binding to cyclin D1 were -21.1 and -24.9 kcal/mol, respectively (). Given theoretical parameters (mfe less than -20 kcal/mol), results suggest that cyclin D1 mRNA is a likely target of both microRNAs in T. s. elegans. In addition to the thermodynamics of microRNA:target hybridization, imperfect complementarity was discovered between both microRNA-16-1 and microRNA-15a binding to cyclin D1, suggesting a mechanism of translational silencing and not target degradation.
MicroRNA-16-1 and microRNA-15a expression.
Using a newly developed microRNA RT-PCR protocol,Citation40 the expression of both microRNA-16-1 and microRNA-15a were assessed in liver, kidney and skeletal muscle of T. s. elegans from control, 5 h anoxic and 20 h anoxic conditions. Both microRNA-16-1 () and microRNA-15a () levels showed an inverse correlation with cyclin D1 protein levels in liver and kidney. Expression levels of microRNA-16-1 increased significantly in liver by 2.58 ± 0.18-fold after 5 h anoxic submergence and by 3.11 ± 0.13-fold after 20 h anoxia as compared with control values (p < 0.05). Similar responses were seen in kidney; microRNA-16-1 levels increased significantly by 1.36 ± 0.03-fold and 1.54 ± 0.07-fold after 5 and 20 h of anoxia, respectively, as compared with controls (p < 0.05). No significant changes in microRNA-16-1 expression were detected in skeletal muscle in response to anoxia. MicroRNA-15a levels also increased significantly in liver by 1.60 ± 0.04-fold after 5 h anoxia and 2.31 ± 0.15-fold after 20 h anoxia as compared with controls (p < 0.05). In kidney, a significant change in microRNA-15a expression occurred only after 20 h anoxia; levels increased by 1.61 ± 0.12-fold as compared with controls (p < 0.05). No significant changes in microRNA-15a expression were found in skeletal muscle tissue in response to anoxic exposure.
Discussion
Previous research on the regulation of cell proliferation has identified cyclin D1 as a crucial component to the initiation of the cell cycle.Citation26 As such, the protein expression of cyclin D1 is tightly coordinated within each phase of the cell cycle, and cyclin D1 translation occurs immediately before it is required. Many studies conclude that an increase in cyclin D1 protein levels is indicative of an increased proliferative state and entrance of cells into the G1 phase of the cell cycle.Citation41,Citation42 Similarly, other studies have shown that a reduction of cyclin D1 protein is a marker of reduced proliferation and cell cycle arrest.Citation42,Citation43 Cell cycle arrest has been hypothesized to be part of the hypometabolic response of turtles to anoxia exposure and was proposed to include strict regulation of cyclin D1 protein expression as well as several inhibitors of the cell cycle (p16INK4a and p27KIP1).Citation22 The findings of this study support this hypothesis.
The responses to anoxia exposure by cyclin D1 protein levels were similar in proliferative tissues (liver and kidney); in both cases, total protein levels decreased significantly to 50–67% of the corresponding aerobic control values (). However, protein levels of cyclin D1 did not change during anoxia in skeletal muscle. This may be a reflection of muscle’s proliferative senescence, perhaps imposed by non-reversible mechanisms of cell cycle arrest, such as that imposed by heterochromatin formation.Citation44 The nuclear presence of cyclin D1 followed a similar trend to total cyclin D1 protein, with levels reduced to 63–68% of control values in both liver and kidney from anoxia-exposed turtles (again with no significant changes in skeletal muscle). Interestingly, the expression of cyclin D1 mRNA did not correlate with protein expression and did not change in response to anoxia exposure in any tissue ().
A lack of correlation between protein and mRNA levels is not uncommon and often results from either controls on protein degradation or post-transcriptional regulation (e.g., translational silencing, mRNA degradation). Indeed, an increased rate of proteasomal degradation of cyclin D1 under anoxia could contribute to the net reduction in protein levels seen in liver and kidney of anoxic turtles. Studies have reported that the degradation of cyclin D1 is dependent on an initial phosphorylation of threonine 268.Citation42,Citation45 This phosphorylation is necessary to initiate poly-ubiquitination and subsequent proteasomal degradation. However, analysis of the relative phosphorylation state of cyclin D1 (T268) found no significant change in response to anoxia in any of the three tissues examined (). This result, as well as unchanged mRNA levels in anoxic tissues, suggests that the anoxia-responsive decrease in cyclin D1 protein levels may be due to reduced mRNA translation. To explore this hypothesis, we next analyzed the responses to anoxia by two microRNAs that are known to regulate cyclin D1 expression.
It has been proposed that the 3’UTR of cyclin D1 is critical in regulating cyclin D1 translation due to a combination of several regulatory elements, including AREs and microRNA binding sites.Citation34,Citation46 Recent studies examining the expression of cyclin D1, with and without the 3’UTR, have demonstrated that deletion of the 3’UTR (and the regulatory sites located within) in human fibroblasts leads to a hyperproliferative state.Citation35 This study further demonstrated that cyclin D1 translation is dependent on the presence of both an AU-rich region and microRNA binding sites. To determine the potential for a similar mode of regulation in T. s. elegans, we sequenced the 3’UTR and analyzed it for the presence of both AU-rich regions and potential microRNA binding sites (). Exploration of the turtle cyclin D1 3’ UTR sequence showed an AU-rich region containing four AUUUA repeats within a ~275 nucleotide region. A number of studies have suggested that the presence of an AU-rich region within the 3’UTR of cyclin D1 leads to an increase in mRNA destabilization and therefore could contribute to a decrease in cyclin D1 mRNA and consequently a decrease in cyclin D1 expression.Citation36,Citation47 However, our data do not suggest mRNA destabilization, since the expression of cyclin D1 mRNA did not change significantly over the anoxic exposure. Indeed, other studies have shown that AU-rich regions in the 3’UTR of cyclin D1 have a stabilizing effect,Citation35 which would agree with our findings. Instead, negative regulation of cyclin D1 translation may be imparted by the presence of a conserved microRNA binding site for microRNA-16-1 and microRNA-15a.
Given the possibility of microRNA regulation of cyclin D1 translation in T. s. elegans, we evaluated the expression of both microRNA-16-1 and microRNA-15a in response to anoxia exposure. The expression profiles of both microRNA species were inversely correlated with the expression of cyclin D1 protein in liver and kidney (). This correlation suggests that a microRNA-induced translational suppression of cyclin D1 could occur under anoxic conditions in these tissues. However, in skeletal muscle, no significant changes occurred in either cyclin D1 protein or microRNA expression. Therefore, the data show that changes in cyclin D1 protein, microRNA-16-1 and microRNA-15a occur in a tissue-specific manner in response to anoxia, probably related to the proliferative potential of the organ, and suggest that the enhanced levels of these microRNAs in anoxic tissues may be an important factor in suppressing translation of cyclin D1 mRNA under anoxic conditions.
MicroRNAs are known to be involved in fine-tuning mRNA expression, regulating both mRNA degradation and translational silencing depending on the degree of binding.Citation48 Important to microRNA binding and regulation is the presence of both a seed region (nearly perfect binding with nucleotides 2–7 on the 5’ end of microRNA) and associated complementary binding (binding with the 3’ end of microRNA). The degree of binding between the two RNA strands dictates the fate of the target mRNA. RNA duplexes displaying perfect Watson-Crick base pairing are cleaved by the endonuclease activity of argonaute (Ago), a member of the microRNA-induced silencing complex (miRISC), while imperfect structures are likely targeted to mRNA storage complexes, including P-bodies and stress granules.Citation48-Citation50 In addition to the degree of complementarity between a microRNA and its target, the thermodynamics of microRNA:target hybridization are critical to the stability of the RNA duplex structure (ΔG° typically less than -20 kcal/mol).Citation51 Theoretical analysis of the conserved microRNA binding site within the cyclin D1 3’UTR, suggests that it is able to bind either microRNA-16-1 or microRNA-15a with a thermodynamically favorable mean ΔG° of -21.1 and -24.9 kcal/mol, respectively (). Additionally, the imperfect binding for both microRNA-16-1 and microRNA-15a suggests a mechanism for translational silencing and not target degradation. This discovery provides a potential mechanism to decrease cyclin D1 protein levels without reducing the expression of cyclin D1 mRNA, such as that seen in the anoxic turtle.
In summary, this study provides the first demonstration of cyclin D1 regulation in the anoxia tolerant turtle, T. s. elegans, and suggests that anoxia-responsive suppression of cyclin D1 protein levels in liver and kidney may be linked to regulation of cyclin D1 transcripts by the actions of microRNA. Taken together with the thermodynamic likelihood of microRNA binding, is study suggests that both microRNA-16-1 and microRNA-15a could have roles in reducing cyclin D1 protein expression through repression of translation during anoxia. Additionally, these microRNAs may have a large influence on the proliferation state of cells in general, as both microRNA-16-1 and microRNA-15a also target multiple other proteins involved in the G1 phase of the cell cycle, including E2F5, cdk4, cdk6 and cyclin E1. Further studies are required to assess the degree of influence exerted by microRNA-16-1 and microRNA-15a on multiple components of the cell cycle during periods of hypometabolism. Given the enormous regulatory potential and significant molecular crosstalk of microRNA, these small regulatory molecules may have widespread effects within metabolically stressed cells. As such, the characteristics of microRNA regulation dovetail with the need for mechanisms of metabolic rate depression to be broadly applicable, readily coordinated, easily induced and readily reversed.Citation3 Gene regulation by microRNA target repression and/or degradation may prove to be a critical component of reversible transitions to and from hypometabolic states.
Materials and Methods
Animal treatments.
Adult female red-eared sliders (Trachemys scripta elegans), 700–1,500 g, were acquired from local suppliers and held at 5 ± 1°C in large plastic tubs (two turtles per tub) filled with dechlorinated tap water for several days before use. Control turtles were sampled from this condition. For anoxia exposure, turtles were transferred to large buckets at 5 ± 1°C that had been previously bubbled with N2 gas for 1 h; 2–3 turtles were added per bucket in 30 min intervals. Bubbling was continued for 1 h after the last turtle was added and was reinitiated again during sampling of the animals. A wire mesh was fitted into the tank about 5 cm below the water surface so that turtles remained submerged throughout the 5 or 20 h experimental anoxic submergence. All animals were killed by decapitation and then liver, kidney and white skeletal muscle were rapidly dissected out, frozen in liquid nitrogen and stored at -80°C until use. All animals were cared for in accordance with the guidelines of the Canadian Council on Animal Care, and all experimental procedures had the prior approval of the Carleton University Animal Care Committee.
Total and nuclear protein isolations.
Samples of frozen tissues (0.5 g) were crushed under liquid nitrogen and then homogenized in 1.25 mL homogenizing buffer (20 mM Hepes pH 7.5, 200 mM NaCl, 0.1 mM EDTA, 10 mM NaF, 1 mM Na3VO4, 10 mM β-glycerophosphate) with the addition of a few crystals of phenylmethylsulfonylfluoride and 1 µL Sigma protease inhibitor cocktail (104 mM AEBSF, 80 µM aprotinin, 4 mM bestatin, 1.4 mM E-64, 2 mM leupeptin, 1.5 mM pepstatin A). Samples were then centrifuged at 4°C for 15 min at 10,000x g. Soluble protein concentrations were assessed using the BioRad protein assay (Cat# 500-0006) with bovine serum albumin as the standard. All samples were then adjusted to a constant concentration of 10 µg/µL by the addition of small amounts of homogenizing buffer. The samples were then mixed 1:1 v:v with 2x SDS loading buffer (100 mM Tris-base, 4% w/v SDS, 20% v/v glycerol, 0.2% w/v bromophenol blue, 10% v/v 2-mercaptoethanol). Final sample concentrations were 5 µg/µL. Proteins were denatured by placing the tubes in boiling water for 5 min. Samples were stored at -80°C until use.
Nuclear extracts were prepared using a slight modification of the method above. Briefly, 0.5 g samples were homogenized using a Dounce homogenizer in 1 mL of homogenization buffer (10 mM Hepes pH 7.9, 10 mM KCl, 10 mM EDTA). A 10 µL aliquot of 100 mM dithiothreitol (DTT) and 10 µL of Sigma protease inhibitor cocktail were added just prior to homogenization. Samples were centrifuged at 10,000x g for 10 min at 4°C, and the supernatant (cytoplasmic extract) was removed. The pellet was resuspended in 150 µL of extraction buffer (20 mM HEPES, 400 mM NaCl; 1 mM EDTA; 10% v/v glycerol). A 1.5 µL aliquot of 100 mM dithiothreitol (DTT) and 1.5 µL of Sigma protease inhibitor cocktail were added just prior to addition of the buffer to the pellet. Tubes containing the samples were put on ice horizontally on a rocking platform for 1 h. Samples were then centrifuged at 10,000x g for 10 min at 4°C. The supernatant (nuclear extract) was collected and extracts were quantified and treated as described above to create samples for western blotting. Final sample concentrations were 5 µg/µL. The integrity of the nuclei was confirmed by immunoblotting of cytoplasmic and nuclear fractions and probing with histone H3 antibody (diluted v:v 1:1,000; Cat# 9715, Cell Signaling).
Western blotting.
Aliquots containing 20 µg protein were loaded onto 10% polyacrylamide gels, together with prestained molecular weight standards (Cat# PM005-0500, FroggaBio) and separated using a discontinuous buffer system. Electrophoresis was performed at 180 V for 45 min using the BioRad Mini-Protean 3 system with 1x Tris-glycine running buffer. Proteins on the gel were then electroblotted onto polyvinylidene difluoride (PVDF) membrane (Cat# IPVH00010, Millipore) using a BioRad mini Trans-Blot cell. The transfer was performed at 160 mA constant amperage for 1.5 h. Following the transfer, membranes were washed in TBST (10 mM Tris, pH 7.5, 150 mM NaCl, 0.05% v/v Tween-20) for 3 x 5 min. The membranes were blocked using 2.5% skimmed milk in TBST for 1 h. After blocking, the membranes were probed with either primary rabbit anti-cyclin D1 antibody (Cat# ab6552, Abcam) or rabbit anti-cyclin D1 (T286) antibody (Cat# ab62151, Abcam). Both primary antibodies were diluted 1:1,000 v:v, and membranes were probed for 24 h at 4°C. The membranes were washed 3 x 5 min with TBST at room temperature and probed with secondary anti-rabbit IgG HRP-linked antibody (1:1,000 v:v dilution) for 1 h (Cat# 7074, Cell Signaling). Membranes were washed again 3 x 5 min in TBST at room temperature and were then developed using enhanced chemiluminescence.
The GeneTools program was used to quantify the protein bands (Syngene). The presence of the appropriate band was confirmed by loading 3 µL of protein ladder to the gel. The cyclin D1 band was found at ~35 kDa. Immunoblotting with α-tubulin-specific antibodies showed constant α-tubulin expression in all experimental conditions and when compared with the combined density of a group of Coomassie stained protein bands. Immunoblots of the proteins of interest were individually adjusted for loading irregularities by normalizing the band intensity of immunoreactive material in each lane against the combined density of a group of Coomassie-stained protein bands in its respective sample lane; these bands showed a similar expression pattern to α-tubulin, and the same stained bands were used for each such comparison.
Total RNA isolation.
Total RNA was isolated from liver and skeletal muscle of turtles using TrizolTM (Cat# 15596-018, Invitrogen). Briefly, 100 mg of tissue was homogenized in 1 mL Trizol using a Polytron homogenizer followed by the addition of 200 µL of chloroform and centrifugation at 10,000x g for 15 min at 4°C. The upper aqueous layer (containing RNA) was removed and placed in a fresh microcentrifuge tube. Total RNA was then precipitated with the addition of 500 µL of isopropanol followed by incubation at room temperature (RT) for 10 min. Samples were then centrifuged as above. The RNA pellet was washed with 70% ethanol and centrifuged again. The supernatant was removed, and tubes were allowed to dry for 10–15 min and then resuspended in 50 µL diethyl pyrocarbonate (DEPC)-treated H2O. RNA quality was assessed by the 260/280 nm ratio as well as gel electrophoresis on a 1% agarose gel stained with 2x Sybr Green I (Cat# S7563; Invitrogen) to check for integrity of the 18S and 28S rRNA bands. All RNA samples were diluted to 1 µg/µL using DEPC-treated ddH2O.
Reverse transcriptase polymerase chain reaction.
A 5 µg aliquot of total RNA from each sample was diluted to 10 µL with DEPC-treated H2O. For measurements of both cyclin D1 and α-tubulin, 1 µL of 200 ng/µL oligo-dT (5’-TTT TTT TTT TTT TTT TTT TTT TV-3’; V = A, G or C) primer was added to each PCR tube. The samples were incubated in a thermal cycler for 5 min at 65°C. The mixture was then chilled rapidly on ice and 4 µL of 5x first strand buffer (Cat# 28025-021, Invitrogen), 2 µL of 0.1 M dithiothreitol (DTT) (Cat# 28025-021, Invitrogen), 1 µL of 25 mM dNTPs (Cat# DD0057, BioBasic) and 1 µL of M-MLV reverse transcriptase (Cat# 28025-021; Invitrogen) were added to each sample. The mixture was placed back into the thermal cycler and incubated for 45 min at 42°C. The resulting cDNA was serial diluted to 10-3 and stored at -20°C. MicroRNA specific reverse transcription was performed as previously described in reference Citation40. A 5.0 µL aliquot of total RNA (1 µg/µL) was incubated with 1.0 µL of 250 nM microRNA-specific stem-loop primer (). The reaction was heated at 95°C for 5 min and then incubated for 5 min at 60°C. After cooling on ice for 1 min, the remaining reagents (4 µL of 5x first strand buffer, 2 µL of 0.1 M DTT, 1 µL of 25 mM dNTPs and 1 µL of M-MLV reverse transcriptase) were added. The reaction proceeded for 30 min at 16°C, followed by 30 min at 42°C and 85°C for 5 min. Following reverse transcription, the product was serial diluted to 10-3 and stored at -20°C.
Table 1. Primers used in this study.
Polymerase chain reaction (PCR) was used to amplify the sequences under study from the cDNA samples. Each PCR reaction consisted of 13.25 µL of sterile water, 5 µL of diluted cDNA, 1.25 µL of 1.5 µM primer mixture (), 2.5 µL of 10x PCR buffer (Cat# N8080006, Invitrogen), 1.5 µL of 50 mM MgCl2, 0.5 µL of 25 mM dNTPs and 1 µL of Taq polymerase, for a total volume of 25 µL. All PCR amplification cycles were as follows: an initial denaturation at 94°C for 7 min, followed by 30–35 cycles of 94°C for 1 min, primer annealing at 60°C for 1 min, and elongation at 72°C for 1 min. The final elongation was at 72°C for 10 min. The 3’ end of cyclin D1 was amplified using the BD Biosciences SMART RACE kit (Cat# 6121, Clontech) following manufacturer’s instructions. The cDNA was amplified using primers listed in . The annealing temperature was 65°C, and an end product of 2,300 bp was obtained and sequenced. MicroRNA-specific RT-PCR was performed with 12.75 µL of sterile water, 5 µL of RT product, 2 µL of 25 µM primer mixture, 2.5 µL of 10x PCR buffer, 1.25 µL of 50 mM MgCl2, 0.5 µL of 25 mM dNTPs and 1 µL of Taq polymerase. The reactions were amplified for 10 sec at 95°C, followed by 30–35 cycles of 95°C for 15 sec and 60°C for 1 min. PCR products were held at 4°C after amplification.
All PCR products were separated on 1–2% agarose gels stained with 2x Sybr Green I nucleic acid gel stain, visualized using the ChemiGenius imaging system (Syngene) under UV light and quantified using the GeneTools program. The bands from the most dilute cDNA sample that gave visible product were used for quantification purposes, ensuring that the products had not reached amplification saturation. The PCR product was sequenced by BioBasic.
Statistics.
Detection of bands on gels and blots used the ChemiGenius Bio-Imaging System (Syngene) and densitometric analysis was performed with the associated Gene Tools software. Immunoblot band intensity in each lane was normalized against a strong Coomassie blue-stained band in the same lane to correct any minor variations in sample loading; the Coomassie stained band chosen showed constant intensity across all samples and was well-separated from the area of the gel containing the immunoreactive protein. For RT-PCR, band intensity was normalized against the corresponding intensity of the α-tubulin (for cyclin D1) or 5S rRNA (for microRNAs) bands amplified from the same RNA sample. Mean normalized band densities ± SEM were calculated for control and anoxic samples and significance testing used one-way analysis of variance followed by the Student-Newman-Keuls test with a significance level of p < 0.05. All data are derived from multiple independent tissue extracts from different animals.
Theoretical predictions of both the conservation of microRNA seed-pairing sites and the thermodynamics of RNA duplex formation were used to determine the ability of both microRNA-16-1 and microRNA-15a to bind the 3’ UTR of cyclin D1. The conservation of both microRNA-16-1 and microRNA-15a seed-pairing was determined using TargetScan 5.2. The TargetScan program searches for microRNA binding sites (seed matches) conserved between several model organisms.Citation52 Thermodynamic analyses were predicted by using RNAhybrid software and a minimum free energy threshold of less than -20.0 kcal/mol.Citation51,Citation53,Citation54
Acknowledgements
Thanks go to J.M. Storey for an editorial review of the manuscript. This work was supported by a Discovery grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada. K.B.S. holds the Canada Research Chair in Molecular Physiology, K.K.B. held an NSERC postgraduate fellowship.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Storey KB. Anoxia tolerance in turtles: metabolic regulation and gene expression. Comp Biochem Physiol A Mol Integr Physiol 2007; 147:263 - 76; http://dx.doi.org/10.1016/j.cbpa.2006.03.019; PMID: 17035057
- Larade K, Storey KB. Living without oxygen: anoxia-responsive gene expression and regulation. Curr Genomics 2009; 10:76 - 85; http://dx.doi.org/10.2174/138920209787847032; PMID: 19794879
- Storey KB, Storey JM. Metabolic rate depression in animals: transcriptional and translational controls. Biol Rev Camb Philos Soc 2004; 79:207 - 33; http://dx.doi.org/10.1017/S1464793103006195; PMID: 15005178
- Jackson DC. Lactate accumulation in the shell of the turtle Chrysemys picta bellii during anoxia at 3°C and 10°C. J Exp Biol 1997; 200:2295 - 300; PMID: 9320212
- Brooks SPJ, Storey KB. Regulation of glycolytic enzymes during anoxia in the turtle Pseudemys scripta.. Am J Physiol 1989; 257:R278 - 83; PMID: 2527474
- Hochachka PW, Land SC, Buck LT. Oxygen sensing and signal transduction in metabolic defense against hypoxia: lessons from vertebrate facultative anaerobes. Comp Biochem Physiol A Physiol 1997; 118:23 - 9; http://dx.doi.org/10.1016/S0300-9629(96)00372-6; PMID: 9243812
- Willmore WG, Storey KB. Antioxidant systems and anoxia tolerance in a freshwater turtle Trachemys scripta elegans.. Mol Cell Biochem 1997; 170:177 - 85; http://dx.doi.org/10.1023/A:1006817806010; PMID: 9144333
- Krivoruchko A, Storey KB. Regulation of the heat shock response under anoxia in the turtle, Trachemys scripta elegans.. J Comp Physiol B 2010; 180:403 - 14; http://dx.doi.org/10.1007/s00360-009-0414-9; PMID: 19834715
- Leung AK, Sharp PA. MicroRNA functions in stress responses. Mol Cell 2010; 40:205 - 15; http://dx.doi.org/10.1016/j.molcel.2010.09.027; PMID: 20965416
- Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell 2007; 27:91 - 105; http://dx.doi.org/10.1016/j.molcel.2007.06.017; PMID: 17612493
- Schier AF, Giraldez AJ. MicroRNA function and mechanism: insights from zebra fish. Cold Spring Harb Symp Quant Biol 2006; 71:195 - 203; http://dx.doi.org/10.1101/sqb.2006.71.055; PMID: 17381297
- Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell 2003; 115:787 - 98; http://dx.doi.org/10.1016/S0092-8674(03)01018-3; PMID: 14697198
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116:281 - 97; http://dx.doi.org/10.1016/S0092-8674(04)00045-5; PMID: 14744438
- Shi Y, Jin Y. MicroRNA in cell differentiation and development. Sci China C Life Sci 2009; 52:205 - 11; http://dx.doi.org/10.1007/s11427-009-0040-5; PMID: 19294345
- Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res 2005; 65:6029 - 33; http://dx.doi.org/10.1158/0008-5472.CAN-05-0137; PMID: 16024602
- Ivey KN, Srivastava D. MicroRNAs as regulators of differentiation and cell fate decisions. Cell Stem Cell 2010; 7:36 - 41; http://dx.doi.org/10.1016/j.stem.2010.06.012; PMID: 20621048
- Biggar KK, Dubuc A, Storey KB. MicroRNA regulation below zero: differential expression of miRNA-21 and miRNA-16 during freezing in wood frogs. Cryobiology 2009; 59:317 - 21; http://dx.doi.org/10.1016/j.cryobiol.2009.08.009; PMID: 19735650
- Morin PJ Jr., Dubuc A, Storey KB. Differential expression of microRNA species in organs of hibernating ground squirrels: a role in translational suppression during torpor. Biochim Biophys Acta 2008; 1779:628 - 33; PMID: 18723136
- Biggar KK, Storey KB. The emerging roles of microRNAs in the molecular responses of metabolic rate depression. J Mol Cell Biol 2011; 3:167 - 75; http://dx.doi.org/10.1093/jmcb/mjq045; PMID: 21177365
- Mazia D. Biochemistry of the dividing cell. Annu Rev Biochem 1962; 30:669 - 88; http://dx.doi.org/10.1146/annurev.bi.30.070161.003321
- Roufayel R, Biggar KK, Storey KB. Regulation of cell cycle components during exposure to anoxia or dehydration stress in the wood frog, Rana sylvatica. J Exp Zool A Ecol Genet Physiol 2011; 315:487 - 94; http://dx.doi.org/10.1002/jez.696; PMID: 21796797
- Biggar KK, Storey KB. Perspectives in cell cycle regulation: lessons from an anoxic vertebrate. Curr Genomics 2009; 10:573 - 84; http://dx.doi.org/10.2174/138920209789503905; PMID: 20514219
- O'Connor C. Cell Division: Stages of Mitosis. Nature Ed. 2008; 1(1).
- Clegg JS. Embryos of Artemia franciscana survive four years of continuous anoxia: the case for complete metabolic rate depression. J Exp Biol 1997; 200:467 - 75; PMID: 9318130
- Douglas RM, Haddad GG. Genetic models in applied physiology: invited review: effect of oxygen deprivation on cell cycle activity: a profile of delay and arrest. J Appl Physiol 2003; 94:2068 - 83, discussion 2084; PMID: 12679355
- Foster DA, Yellen P, Xu L, Saqcena M. Regulation of G1 cell cycle progression: Distinguishing the restriction point from a nutrient-sensing cell growth checkpoint(s). Genes Cancer 2010; 1:1124 - 31; http://dx.doi.org/10.1177/1947601910392989; PMID: 21779436
- Tarn WY, Lai MC. Translational control of cyclins. Cell Div 2011; 6:5; http://dx.doi.org/10.1186/1747-1028-6-5; PMID: 21314915
- Matsushime H, Quelle DE, Shurtleff SA, Shibuya M, Sherr CJ, Kato JY. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol 1994; 14:2066 - 76
- Sherr CJ. D-type cyclins. Trends Biochem Sci 1995; 20:187 - 90; http://dx.doi.org/10.1016/S0968-0004(00)89005-2; PMID: 7610482
- Rosenwald IB, Lazaris-Karatzas A, Sonenberg N, Schmidt EV. Elevated levels of cyclin D1 protein in response to increased expression of eukaryotic initiation factor 4E. Mol Cell Biol 1993; 13:7358 - 63; PMID: 8246956
- Jiang W, Kahn SM, Zhou P, Zhang YJ, Cacace AM, Infante AS, et al. Overexpression of cyclin D1 in rat fibroblasts causes abnormalities in growth control, cell cycle progression and gene expression. Oncogene 1993; 8:3447 - 57; PMID: 8247550
- Courjal F, Louason G, Speiser P, Katsaros D, Zeillinger R, Theillet C. Cyclin gene amplification and overexpression in breast and ovarian cancers: evidence for the selection of cyclin D1 in breast and cyclin E in ovarian tumors. Int J Cancer 1996; 69:247 - 53; http://dx.doi.org/10.1002/(SICI)1097-0215(19960822)69:4<247::AID-IJC1>3.0.CO;2-X; PMID: 8797862
- Hayakawa Y, Hirata Y, Nakagawa H, Sakamoto K, Hikiba Y, Kinoshita H, et al. Apoptosis signal-regulating kinase 1 and cyclin D1 compose a positive feedback loop contributing to tumor growth in gastric cancer. Proc Natl Acad Sci U S A 2011; 108:780 - 5; http://dx.doi.org/10.1073/pnas.1011418108; PMID: 21187402
- Wiestner A, Tehrani M, Chiorazzi M, Wright G, Gibellini F, Nakayama K, et al. Point mutations and genomic deletions in CCND1 create stable truncated cyclin D1 mRNAs that are associated with increased proliferation rate and shorter survival. Blood 2007; 109:4599 - 606; http://dx.doi.org/10.1182/blood-2006-08-039859; PMID: 17299095
- Deshpande A, Pastore A, Deshpande AJ, Zimmermann Y, Hutter G, Weinkauf M, et al. 3’UTR mediated regulation of the cyclin D1 proto-oncogene. Cell Cycle 2009; 8:3584 - 92; http://dx.doi.org/10.4161/cc.8.21.9993; PMID: 19823025
- Lin S, Wang W, Wilson GM, Yang X, Brewer G, Holbrook NJ, et al. Down-regulation of cyclin D1 expression by prostaglandin A(2) is mediated by enhanced cyclin D1 mRNA turnover. Mol Cell Biol 2000; 20:7903 - 13; http://dx.doi.org/10.1128/MCB.20.21.7903-7913.2000; PMID: 11027261
- Liu Q, Fu H, Sun F, Zhang H, Tie Y, Zhu J, et al. miR-16 family induces cell cycle arrest by regulating multiple cell cycle genes. Nucleic Acids Res 2008; 36:5391 - 404; http://dx.doi.org/10.1093/nar/gkn522; PMID: 18701644
- Chen RW, Bemis LT, Amato CM, Myint H, Tran H, Birks DK, et al. Truncation in CCND1 mRNA alters miR-16-1 regulation in mantle cell lymphoma. Blood 2008; 112:822 - 9; http://dx.doi.org/10.1182/blood-2008-03-142182; PMID: 18483394
- Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A 2002; 99:15524 - 9; http://dx.doi.org/10.1073/pnas.242606799; PMID: 12434020
- Biggar KK, Kornfeld SF, Storey KB. Amplification and sequencing of mature microRNAs in uncharacterized animal models using stem-loop reverse transcription-polymerase chain reaction. Anal Biochem 2011; 416:231 - 3; http://dx.doi.org/10.1016/j.ab.2011.05.015; PMID: 21651887
- Stacey DW. Cyclin D1 serves as a cell cycle regulatory switch in actively proliferating cells. Curr Opin Cell Biol 2003; 15:158 - 63; http://dx.doi.org/10.1016/S0955-0674(03)00008-5; PMID: 12648671
- Masamha CP, Benbrook DM. Cyclin D1 degradation is sufficient to induce G1 cell cycle arrest despite constitutive expression of cyclin E2 in ovarian cancer cells. Cancer Res 2009; 69:6565 - 72; http://dx.doi.org/10.1158/0008-5472.CAN-09-0913; PMID: 19638577
- Nishi K, Inoue H, Schnier JB, Rice RH. Cyclin D1 downregulation is important for permanent cell cycle exit and initiation of differentiation induced by anchorage-deprivation in human keratinocytes. J Cell Biochem 2009; 106:63 - 72; http://dx.doi.org/10.1002/jcb.21978; PMID: 19021145
- Zhang R, Adams PD. Heterochromatin and its relationship to cell senescence and cancer therapy. Cell Cycle 2007; 6:784 - 9; http://dx.doi.org/10.4161/cc.6.7.4079; PMID: 17377503
- Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer 2007; 6:24; http://dx.doi.org/10.1186/1476-4598-6-24; PMID: 17407548
- Rimokh R, Berger F, Bastard C, Klein B, French M, Archimbaud E, et al. Rearrangement of CCND1 (BCL1/PRAD1) 3′ untranslated region in mantle-cell lymphomas and t(11q13)-associated leukemias. Blood 1994; 83:3689 - 96; PMID: 8204893
- Guo Y, Harwalkar J, Stacey DW, Hitomi M. Destabilization of cyclin D1 message plays a critical role in cell cycle exit upon mitogen withdrawal. Oncogene 2005; 24:1032 - 42; http://dx.doi.org/10.1038/sj.onc.1208299; PMID: 15592507
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009; 136:215 - 33; http://dx.doi.org/10.1016/j.cell.2009.01.002; PMID: 19167326
- Liu J, Valencia-Sanchez MA, Hannon GJ, Parker R. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat Cell Biol 2005; 7:719 - 23; http://dx.doi.org/10.1038/ncb1274; PMID: 15937477
- Leung AKL, Calabrese JM, Sharp PA. Quantitative analysis of Argonaute protein reveals microRNA-dependent localization to stress granules. Proc Natl Acad Sci U S A 2006; 103:18125 - 30; http://dx.doi.org/10.1073/pnas.0608845103; PMID: 17116888
- Singh J, Nagaraju J. In silico prediction and characterization of microRNAs from red flour beetle (Tribolium castaneum). Insect Mol Biol 2008; 17:427 - 36; http://dx.doi.org/10.1111/j.1365-2583.2008.00816.x; PMID: 18651924
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005; 120:15 - 20; http://dx.doi.org/10.1016/j.cell.2004.12.035; PMID: 15652477
- Rehmsmeier M, Steffen P, Hochsmann M, Giegerich R. Fast and effective prediction of microRNA/target duplexes. RNA 2004; 10:1507 - 17; http://dx.doi.org/10.1261/rna.5248604; PMID: 15383676
- Watanabe Y, Yachie N, Numata K, Saito R, Kanai A, Tomita M. Computational analysis of microRNA targets in Caenorhabditis elegans.. Gene 2006; 365:2 - 10; http://dx.doi.org/10.1016/j.gene.2005.09.035; PMID: 16356665