Abstract
Multiple myeloma (MM) is a progressive malignant disorder characterized by accumulation of plasma cells in the bone marrow. MM remains an incurable disease with a 5-y survival rate of approximately 40%. While clinical response rates to first line chemotherapeutics are high, disease relapse is inevitable, and occurs because a small fraction of the original myeloma cells appear to be resistant to treatment. Heme oxygenase-1 (HO-1) is an Nrf2 transcription factor-regulated gene that is commonly induced following oxidative stress and cellular injury, functioning to decrease oxidative stress and inflammatory responses, protecting against apoptosis and altering the cell cycle. We and others have highlighted the role of HO-1 in providing cellular protection against chemotherapeutic drugs in a number of cancer cells, which we have highlighted here in this Extra View. Furthermore, we explored the expression of HO-1 in multiple myeloma cells in response to the key anti-myeloma drugs bortezomib and lenalidomide. We show here for the first time that bortezomib increases HO-1 expression in a time- and concentration-dependent manner. Moreover, we also observe that HO-1 is increased in lenalidomide-resistant MM cell lines. Altogether, we highlight a possible role for HO-1 in basal and acquired chemoresistance in MM.
Keywords: :
Introduction
Multiple myeloma (MM) is a malignant neoplasm of plasma cells and represents about 10% of hematologic malignancies and 1% of all cancers. Across Europe, there are approximately 21,000 new patients per year diagnosed with MM and 16,000 deaths per year from the disease.Citation1 A number of significant therapeutic advances have been made in the past 15 years, including the proteasome inhibitor bortezomib as well as thalidomide and its derivative lenalidomide, which have been associated with improved survival outcomes for patients with MM.Citation2,Citation3 However, despite these developments in treatment, relapse is still inevitable, and MM remains an incurable disease with a 5-y survival of about 40%. As such, considerable effort is currently being invested in developing a better understanding of myeloma biology with a view to overcoming chemotherapy failure with the ultimate goal of achieving strategies that will reliably cure MM.
One of the main characteristics of cancer cells, when compared with their parental normal counterparts, is a continuous pro-oxidant state that can lead to intrinsic oxidative stress. For instance, primary chronic lymphocytic leukemia cells have been shown to have increased reactive oxygen species (ROS) production when compared with normal lymphocytes.Citation4 This persistent ROS stress may induce adaptive stress responses, allowing cancer cells to survive with elevated levels of ROS and preserve cellular viability. This aberrantly activated intracellular ROS-scavenging system could have detrimental effects on anticancer drugs that work through accumulation of ROS to stimulate cytotoxicity and cell death.Citation5
In this respect, heme oxygenase-1 (HO-1) is an enzyme that has recently come to light as playing a potentially central role in cancer cell survival. HO-1 is part of a family of heme oxygenases (HO), which are enzymes that catalyze the initial rate-limiting step of heme degradation to generate biliverdin, free heme iron and carbon monoxide. The classical physiological functions of HO are to decrease oxidative stress and inflammatory responses and to protect against apoptosis by the removal of heme, a potent pro-oxidant and pro-inflammatory mediator. Two major isoforms of HO have been identified in human: an inducible isoform, HO-1 (also named heat shock protein 32), and a constitutively expressed isoform, HO-2. HO-1 is regulated by the Nrf2/antioxidant response element (ARE) located in the upstream promoter region of HO-1, and its mRNA and protein expression are commonly upregulated following oxidative stress and cellular injury.Citation6-Citation8 Other mechanisms of HO-1 transcriptional regulation have also been described through the hemoprotein Bach1, a heme-sensing protein that binds to and inhibits Maf proteins, the crucial heterodimer partners that are necessary for Nrf2 to bind to ARE.Citation9-Citation11 In addition, we and others have shown that NFκB and AP-1 can also regulate HO-1 transcription.Citation12,Citation13 Moreover, Kirino and colleagues have shown that the pro-inflammatory cytokine tumor necrosis factorα (TNF) can induce downregulation of HO-1 in human monocytes by promoting the degradation of HO-1 mRNA.Citation14
Taken together, we now increasingly understand that while HO-1 is physiologically regulated by multiple mechanisms, there are a variety of cancer cell types which have hijacked the HO-1 regulatory pathways to utilize this protein to protect against chemotherapy-induced increases in ROS and, thus, to promote tumor survival.
This review will highlight the major findings from studies of HO-1 in cancer and discuss their significance in the context of known HO-1 activation pathways. In addition to reviewing recent work exploring the role of HO-1 in chemotherapy resistance, we will discuss novel insights into the role of HO-1 induction in multiple myeloma cells and implications for future therapies.
HO-1 in Hematological Malignancies
Aberrant expression of HO-1 in human cancers is implicated in oncogenesis and chemoresistance.Citation15-Citation17 highlights the major studies showing the effect of HO-1 in hematologic malignancies.
Table 1. HO-1 functional expression in hematological malignancies.
Work performed in chronic myeloid leukemia (CML) has shown that primary CML cells and the CML-derived K562 cell line constitutively expresses HO-1, and, furthermore, HO-1 has been identified as a BCR/ABL oncoprotein-dependent survival factor.Citation18 Moreover, numerous studies have revealed that HO-1 provides resistance to imatinib, which inhibits the tyrosine kinase BCR-ABL, and that HO-1 silencing returns the cells to the imatinib-sensitive phenotype.Citation19,Citation20
Our own investigations have shown that in AML-derived cells, but not primary cells, HO-1 is upregulated in response to TNF stimulation in conjunction with NFκB inhibition.Citation21 Furthermore, this induction of HO-1 protects AML cells from cell death signals. The signaling machinery involved in regulating this response is Nrf2. HO-1 and Nrf2 may protect against the detrimental effects of inflammation and oxidative stress but may also help protect cancerous AML cells from TNF-mediated cell death, resulting in potentially clinically significant levels of apoptosis resistance. Moreover, our work and a study by Ishigatsubo and colleagues have revealed that Nrf2 is constitutively active in some AML samples but not in others.Citation5,Citation22 This heterogeneity in the levels of Nrf2 observed in AML samples could be the result of acquired somatic mutations in Keap1 or Nrf2 or as a consequence of epigenetic changes that can deregulate the expression of these genes.Citation23,Citation24 In addition, the study by Miyazaki et al. showed that constitutive Nrf2 induces HO-1, which provides protection for AML cells from chemotherapy-induced apoptosis.Citation22 More recently, another study has confirmed our own findings that inhibiting HO-1 activity with zinc protoporphyrin induces apoptosis in AML cells,Citation25 suggesting that induced HO-1 expression plays a protective role in this disease, and that HO-1 is a candidate drugable target.
In lymphocytic leukemias little is known about the role of HO-1, with the possible exception of one study in which HO-1 was shown to be increased in chronic lymphocytic leukemia.Citation26 To further understand the role of HO-1 in protecting MM cells from apoptosis we describe here the expression profile of HO-1 in MM primary cells and cell lines in response to lenalidomide and bortezomib over a short (4 and 8 h) and long (12 weeks) treatment period.
Multiple Myeloma and HO-1 Chemotherapy-Induced Expression
To date, the role of HO-1 in MM has not been examined with regards to current chemotherapies. Here, we show for the first time that the clinically used MM chemotherapeutic drug bortezomib, but not lenalidomide, induces HO-1 expression over an 8-h time course (). Our data show that HO-1 is upregulated in the MM primary cells used and cell lines tested, with the exception of the U266 cell line in which HO-1 was undetected. In addition, the dose-dependent nature of these drugs on HO-1 expression in RPMI8226 cells are presented ().
Figure 1. Bortezomib induces HO-1 mRNA expression in MM. (A) MM primary cells and cell lines were treated with bortezomib and lenalidamide for 4 and 8 h and then total RNA was extracted. RNA was reverse transcribed and relative expression of HO-1 mRNA was measured by real-time PCR. (B) RPMI8226 cells were treated for 8 h with various doses of bortezomib and lenalidamide. Total RNA was extracted and reverse transcribed. Relative expression of HO-1 mRNA was measured by real-time PCR. Data represent the means ± SEM, from three separate experiments.
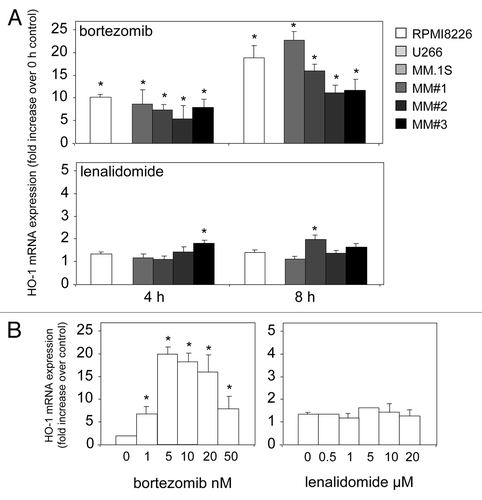
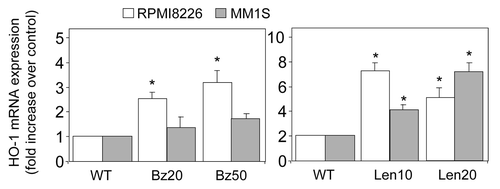
HO-1 overexpression has also been described in relapse or recurrent malignancies.Citation27 To explore the molecular basis of acquired resistance to bortezomib and lenalidomide, MM cell lines RPMI8226 and MM1S cells were exposed in vitro over a period of 3 mo to step-wise increasing concentrations of bortezomib (1 nM to 50 nM) and lenalidomide (500 nM to 20 μM). RPMI8226 and MM1S cells grown in the presence of 20 and 50 nM bortezomib (Bz20 and Bz50), 10 and 20 μM lenalidomide (Len10 and Len20) were used for characterization.
Next, we wanted to determine the expression of HO-1 in bortezomib- and lenalidomide-resistant MM cells. This was done to mimic the in vivo relapse or chemotherapy-resistant nature of the disease. shows that in lenalidomide-resistant MM cells, HO-1 expression has increased in both RPMI8226 and MM1S when compared with non-resistant cells. In contrast, the results for the increased expression of HO-1 in bortezomib-resistant MM1S cells were not significant. However, a slight but significant increase in HO-1 was observed in bortezomib-resistant RPMI8226 cells when compared with non-resistant cells. These results suggest that MM cells are capable of increasing the levels of HO-1 in order to protect against increased ROS and, therefore, lower the damaging effect of these chemotherapies in this disease. Thus, it is not inconceivable to propose that increased HO-1 in MM provides protection against the initial hit of bortezomib treatment () and, in the long-term, protection against lenalidomide treatment ().
Figure 2. Expression of HO-1 in chemotherapy resistant MM cell lines. MM cell lines RPMI8226 and MM1S cells were treated for 3 mo with increasing doses of bortezomib (up to 50 nM) or lenalidomide (up to 20 µM). Total RNA was extracted and reverse transcribed. Relative expression of HO-1 mRNA was measured by real-time PCR. Data represent the means ± SEM, from three separate experiments.
Concluding Remarks
In conclusion, HO-1 has been implicated as a significant chemo-resistance pathway in several hematological malignancies. Myeloma can now be added to this list. Work is ongoing to establish whether these in vitro findings can be translated into drug and trial design strategies with the overall goal of achieving improved outcomes for patients with these diseases.
| Abbreviations: | ||
| MM | = | multiple myeloma |
| HO-1 | = | heme oxygenase-1 |
| ARE | = | antioxidant responsive element |
| ROS | = | reactive oxygen species |
Acknowledgments
This study was supported by research funding in the form of grant support from the National Institutes of Health Research (NIHR), The Big C, Association for International Cancer Research (AICR) and Leukaemia and Lymphoma Research (LLR).
References
- Harousseau J-L, Dreyling M, ESMO Guidelines Working Group. Multiple myeloma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2008; 19:Suppl 2 ii55 - 7; http://dx.doi.org/10.1093/annonc/mdn088; PMID: 18456769
- Dimopoulos MA, Richardson PG, Brandenburg N, Yu Z, Weber DM, Niesvizky R, et al. A review of second primary malignancy in patients with relapsed or refractory multiple myeloma treated with lenalidomide. Blood 2012; 119:2764 - 7; http://dx.doi.org/10.1182/blood-2011-08-373514; PMID: 22323483
- Richardson PG, Laubach JP, Schlossman RL, Ghobrial IM, Redman KC, McKenney M, et al. The potential benefits of participating in early-phase clinical trials in multiple myeloma: long-term remission in a patient with relapsed multiple myeloma treated with 90 cycles of lenalidomide and bortezomib. Eur J Haematol 2012; 88:446 - 9; http://dx.doi.org/10.1111/j.1600-0609.2012.01765.x; PMID: 22300348
- Zhou Y, Hileman EO, Plunkett W, Keating MJ, Huang P. Free radical stress in chronic lymphocytic leukemia cells and its role in cellular sensitivity to ROS-generating anticancer agents. Blood 2003; 101:4098 - 104; http://dx.doi.org/10.1182/blood-2002-08-2512; PMID: 12531810
- Rushworth SA, Bowles KM, MacEwan DJ. High basal nuclear levels of Nrf2 in acute myeloid leukemia reduces sensitivity to proteasome inhibitors. Cancer Res 2011; 71:1999 - 2009; http://dx.doi.org/10.1158/0008-5472.CAN-10-3018; PMID: 21212410
- Schäfer M, Dütsch S, auf dem Keller U, Werner S. Nrf2: a central regulator of UV protection in the epidermis. Cell Cycle 2010; 9:2917 - 8; http://dx.doi.org/10.4161/cc.9.15.12701; PMID: 20699662
- Alam J, Stewart D, Touchard C, Boinapally S, Choi AMK, Cook JL. Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem 1999; 274:26071 - 8; http://dx.doi.org/10.1074/jbc.274.37.26071; PMID: 10473555
- Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, et al. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem 2000; 275:16023 - 9; http://dx.doi.org/10.1074/jbc.275.21.16023; PMID: 10821856
- Villeneuve NF, Sun Z, Chen W, Zhang DD. Nrf2 and p21 regulate the fine balance between life and death by controlling ROS levels. Cell Cycle 2009; 8:3255 - 6; http://dx.doi.org/10.4161/cc.8.20.9565; PMID: 19806015
- Sun J, Hoshino H, Takaku K, Nakajima O, Muto A, Suzuki H, et al. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J 2002; 21:5216 - 24; http://dx.doi.org/10.1093/emboj/cdf516; PMID: 12356737
- Dhakshinamoorthy S, Jain AK, Bloom DA, Jaiswal AK. Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H:quinone oxidoreductase 1 gene expression and induction in response to antioxidants. J Biol Chem 2005; 280:16891 - 900; http://dx.doi.org/10.1074/jbc.M500166200; PMID: 15734732
- Lavrovsky Y, Schwartzman ML, Levere RD, Kappas A, Abraham NG. Identification of binding sites for transcription factors NF-κ B and AP-2 in the promoter region of the human heme oxygenase 1 gene. Proc Natl Acad Sci USA 1994; 91:5987 - 91; http://dx.doi.org/10.1073/pnas.91.13.5987; PMID: 8016102
- Rushworth SA, Bowles KM, Raninga P, MacEwan DJNF. NF-kappaB-inhibited acute myeloid leukemia cells are rescued from apoptosis by heme oxygenase-1 induction. Cancer Res 2010; 70:2973 - 83; http://dx.doi.org/10.1158/0008-5472.CAN-09-3407; PMID: 20332229
- Kirino Y, Takeno M, Murakami S, Kobayashi M, Kobayashi H, Miura K, et al. Tumor necrosis factor alpha acceleration of inflammatory responses by down-regulating heme oxygenase 1 in human peripheral monocytes. Arthritis Rheum 2007; 56:464 - 75; http://dx.doi.org/10.1002/art.22370; PMID: 17265482
- Sunamura M, Duda DG, Ghattas MH, Lozonschi L, Motoi F, Yamauchi J, et al. Heme oxygenase-1 accelerates tumor angiogenesis of human pancreatic cancer. Angiogenesis 2003; 6:15 - 24; http://dx.doi.org/10.1023/A:1025803600840; PMID: 14517400
- Rushworth SA, Zaitseva L, Langa S, Bowles KM, MacEwan DJ. FLIP regulation of HO-1 and TNF signalling in human acute myeloid leukemia provides a unique secondary anti-apoptotic mechanism. Oncotarget 2010; 1:359 - 66; PMID: 21307400
- Heasman SA, Zaitseva L, Bowles KM, Rushworth SA, MacEwan DJ. Protection of acute myeloid leukaemia cells from apoptosis induced by front-line chemotherapeutics is mediated by haem oxygenase-1. Oncotarget 2011; 2:658 - 68; PMID: 21911919
- Mayerhofer M, Florian S, Krauth MT, Aichberger KJ, Bilban M, Marculescu R, et al. Identification of heme oxygenase-1 as a novel BCR/ABL-dependent survival factor in chronic myeloid leukemia. Cancer Res 2004; 64:3148 - 54; http://dx.doi.org/10.1158/0008-5472.CAN-03-1200; PMID: 15126353
- Rakshit S, Bagchi J, Mandal L, Paul K, Ganguly D, Bhattacharjee S, et al. N-acetyl cysteine enhances imatinib-induced apoptosis of Bcr-Abl+ cells by endothelial nitric oxide synthase-mediated production of nitric oxide. Apoptosis 2009; 14:298 - 308; http://dx.doi.org/10.1007/s10495-008-0305-7; PMID: 19153832
- Mayerhofer M, Gleixner KV, Mayerhofer J, Hoermann G, Jaeger E, Aichberger KJ, et al. Targeting of heat shock protein 32 (Hsp32)/heme oxygenase-1 (HO-1) in leukemic cells in chronic myeloid leukemia: a novel approach to overcome resistance against imatinib. Blood 2008; 111:2200 - 10; http://dx.doi.org/10.1182/blood-2006-11-055723; PMID: 18024796
- Rushworth SA, MacEwan DJ. HO-1 underlies resistance of AML cells to TNF-induced apoptosis. Blood 2008; 111:3793 - 801; http://dx.doi.org/10.1182/blood-2007-07-104042; PMID: 18202225
- Miyazaki T, Kirino Y, Takeno M, Samukawa S, Hama M, Tanaka M, et al. Expression of heme oxygenase-1 in human leukemic cells and its regulation by transcriptional repressor Bach1. Cancer Sci 2010; 101:1409 - 16; http://dx.doi.org/10.1111/j.1349-7006.2010.01550.x; PMID: 20345481
- Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci 2009; 34:176 - 88; http://dx.doi.org/10.1016/j.tibs.2008.12.008; PMID: 19321346
- Wang R, An J, Ji F, Jiao H, Sun H, Zhou D. Hypermethylation of the Keap1 gene in human lung cancer cell lines and lung cancer tissues. Biochem Biophys Res Commun 2008; 373:151 - 4; http://dx.doi.org/10.1016/j.bbrc.2008.06.004; PMID: 18555005
- Herrmann H, Kneidinger M, Cerny-Reiterer S, Rülicke T, Willmann M, Gleixner KV, et al. The Hsp32 inhibitors SMA-ZnPP and PEG-ZnPP exert major growth-inhibitory effects on D34(+)/CD38(+) and CD34(+)/CD38(-) AML progenitor cells. Curr Cancer Drug Targets 2012; 12:51 - 63; http://dx.doi.org/10.2174/156800912798888992; PMID: 22165967
- Wu RP, Hayashi T, Cottam HB, Jin G, Yao S, Wu CCN, et al. Nrf2 responses and the therapeutic selectivity of electrophilic compounds in chronic lymphocytic leukemia. Proc Natl Acad Sci USA 2010; 107:7479 - 84; http://dx.doi.org/10.1073/pnas.1002890107; PMID: 20368435
- Li Y, Su J, DingZhang X, Zhang J, Yoshimoto M, Liu S, et al. PTEN deletion and heme oxygenase-1 overexpression cooperate in prostate cancer progression and are associated with adverse clinical outcome. J Pathol 2011; 224:90 - 100; http://dx.doi.org/10.1002/path.2855; PMID: 21381033
- Singh MM, Irwin ME, Gao Y, Ban K, Shi P, Arlinghaus RB, et al. Inhibition of the NADPH oxidase regulates heme oxygenase 1 expression in chronic myeloid leukemia. Cancer 2011; In press http://dx.doi.org/10.1002/cncr.26621; PMID: 22139798
- Zhou P, Kalakonda N, Comenzo RL. Changes in gene expression profiles of multiple myeloma cells induced by arsenic trioxide (ATO): possible mechanisms to explain ATO resistance in vivo. Br J Haematol 2005; 128:636 - 44; http://dx.doi.org/10.1111/j.1365-2141.2005.05369.x; PMID: 15725085
- Bancos S, Baglole CJ, Rahman I, Phipps RP. Induction of heme oxygenase-1 in normal and malignant B lymphocytes by 15-deoxy-Δ(12,14)-prostaglandin J(2) requires Nrf2. Cell Immunol 2010; 262:18 - 27; http://dx.doi.org/10.1016/j.cellimm.2009.12.003; PMID: 20064636