Abstract
Comment on: Buontempo F, et al. Cell Cycle 2012; 11:2467-75.
The Notch signaling pathway is highly conserved and plays an important role in the regulation of cellular proliferation, differentiation and apoptosis. Constitutive activation of Notch signaling results in excessive cellular proliferation and a wide range of malignancies. However, Notch can also act as a tumor suppressor, and its inactivation has been associated with an increased risk of spontaneous squamous cell carcinoma.Citation1 Signaling through the Notch1 receptor is essential for normal T-cell fate specification as well as thymocyte maturation.Citation2 Approximately 50% of human T-ALLs display activating Notch1 mutations, suggesting an important pathogenetic role for Notch1 in T-ALL.Citation3 Activating mutations identified in the T-ALL cluster at the heterodimerization domain (HD) and the proline, glutamine, serine and threonine (PEST) NICD1, respectively.
NFκBs are sequestered in the cytoplasm by the members of the IκB family (IκB-α, IκB-β and IκB-γ). When the IKK protein kinase complex signalosome, phosphorylates IκB, NFκB translocates to the nucleus, dimerizes and engages in the transcription of downstream genes. NFκB-signaling cross-talks with Notch at multiple levels. NFκB signaling results in increased expression of Notch receptors and ligands, leading to augmented Notch signaling,Citation4 and, conversely, activated Notch signaling upregulates expression of NFκB members.Citation5 The NFκB pathway is constitutively activated in human T-ALL cells that harbor Notch1 mutations. Moreover, it has been documented that IKK/NFκB signaling is essential for the maintenance of T-ALL, as leukemic cells that are unable to activate the IKK kinase complex rapidly enter apoptosis. Hence, the NFκB pathway is a potential molecular target for the treatment of T-ALL.Citation6 Accordingly, in the study by Buontempo et al. in this issue of Cell Cycle, the authors report the anti-proliferative effects induced by BMS-345541 (a highly selective IKK inhibitor) in three Notch1-mutated T-ALL cell lines and in T-ALL primary cells from pediatric patients. BMS-345541 induced apoptosis and accumulation of cells in the G2/M phase of the cell cycle via inhibition of IKK/NFκB signaling. Interestingly, they also showed that T-ALL cells treated with BMS-345541 displayed nuclear translocation of FOXO3a and restoration of its functions, including control of p21Cip1 expression levels.
The human FOXO transcription factor family upregulates genes involved in the control of the cell cycle (p27Kip1 and p21Cip1) or in the induction of apoptosis. FOXO3a overexpression inhibits tumor growth in vitro and tumor size in vivo in breast cancer cells. Cytoplasmic location of FOXO3a correlates with poor survival in breast cancer patients.Citation7 The adverse prognostic value of highly phosphorylated FOXO3a and its cytoplasmic sequestration in acute myelogenous leukemia (AML) have been reported. In tumor cells, AKT, IKK and ERK 1/2 control FOXO3a activation through phosphorylation at different amino acidic residues, thus inducing its translocation from the nucleus to the cytoplasm and its subsequent degradation. Akt regulates the subcellular localization of FOXO3a by phosphorylation, thereby preventing the protein from translocating to the nucleus and regulating transcription. Constitutive Akt-activation is frequently correlated with cytoplasmatic FOXO3a in breast tumors, and this is associated with decreased patient survival. Nonetheless, FOXO3a is found in the cytoplasm in the absence of activated Akt. Strikingly, IKKβ interacts with and phosphorylates FOXO3a.Citation8 In the current study, the authors demonstrate that FOXO3a subcellular redistribution is independent of AKT and ERK1/2 signaling. Constitutive phosphorylation of IKK on Ser176/178, which reflects its catalytic activity, is also detectable in the T-ALL models. By using BMS-345541 and also a peptide that directly targets oligomerization of NEMO protein, the authors observed the apoptotic effects and restoration of FOXO3a tumor suppressor functions, regardless of AKT and ERK 1/2 activity. They speculate that in T-ALL the loss of FOXO3a tumor suppressor function could be due to deregulation of IKK, as has been previously demonstrated in other cancer types. It is well known that, differently from p53, FOXO3a mutations have not yet been found in human tumors, which makes therapeutics activating FOXO3a more appealing than others. For these features, BMS-345541 could be used alone or in combination with traditional therapies in the treatment of T-ALL. Hence, this exciting study suggests that the IKKs might serve as a potential drug target in anticancer therapy, since multiple signal transduction pathways inhibiting proliferation and facilitating cell death could be activated. ()
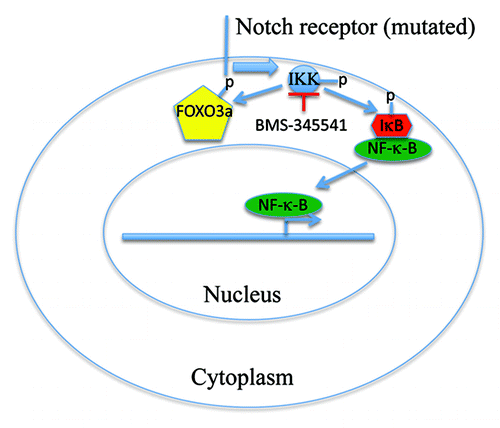
Figure 1. Schematic model depicting the role of IKK inhibition in regulating FOXO3a and NFκB nuclear translocation. In T-ALL, when Notch is mutated and constitutive active, IKK is constant active, which phosphorylates IκB and elicits NFκB nuclear translocation and promote tumorigenesis. The active IKK also sequesters FOXO3a in the cytoplasm. Inhibition of IKK by BMS-345541 triggers FOXO3a nuclear translocation and induces cell cycle arrest and apoptosis.
References
- Pancewicz J, et al. BMC Cancer 2011; 11:502; http://dx.doi.org/10.1186/1471-2407-11-502; PMID: 22128846
- Koch U, et al. Trends Immunol 2011; 32:434 - 42; http://dx.doi.org/10.1016/j.it.2011.06.005; PMID: 21775206
- Weng AP, et al. Science 2004; 306:269 - 71; http://dx.doi.org/10.1126/science.1102160; PMID: 15472075
- Moran ST, et al. J Immunol 2007; 179:195 - 200; PMID: 17579038
- Cheng P, et al. J Immunol 2001; 167:4458 - 67; PMID: 11591772
- Aifantis I, et al. Nat Rev Immunol 2008; 8:380 - 90; http://dx.doi.org/10.1038/nri2304; PMID: 18421304
- Yang JY, et al. Clin Cancer Res 2009; 15:752 - 7; http://dx.doi.org/10.1158/1078-0432.CCR-08-0124; PMID: 19188143
- Hu MC, et al. Cell 2004; 117:225 - 37; http://dx.doi.org/10.1016/S0092-8674(04)00302-2; PMID: 15084260