Malfunction of blood vessels and abnormal vessel growth are associated with various medical conditions ranging from heart disease, cancer and metabolic problems, such as diabetes, to age-related conditions, such as macular degeneration. During normal development or regeneration of adult tissues, newly formed blood vessels undergo maturation. It is important to understand how this process occurs on a cellular and molecular level, because its failure leads to structural and functional deficiencies of blood vessels. Tumor blood vessels are highly permeable, tortuous, dilated and saccular and poorly covered by mural cells (pericytes).Citation1 These properties, which are primarily attributed to impaired tumor vessel maturation, underlie inadequate blood circulation and poor oxygenation of tumors. Although these anomalies could potentially slow tumor growth, they also reduce the sensitivity of tumors to ionizing radiation and impede delivery of chemotherapeutic agents, allowing tumors to become resistant to cytotoxic therapies.Citation2 Excessive vessel permeability associated with tumors also causes clinical complications, such as cerebral edema, in brain cancer patients. The ability to control vessel maturity in tumors, therefore, provides a potential therapeutic opportunity.
Recently, we reported a key role for R-Ras in promoting maturation and normalization of tumor blood vessels.Citation3 R-Ras is a small GTPase of the Ras family with antiangiogenic activity.Citation4 We found that malformation and malfunction of tumor vessels are exacerbated by genetic disruption of R-Ras.Citation3 R-Ras-deficient vessels exhibited severely impaired pericyte and basement membrane supports, disrupted adherens junctions and excessive blood leakage in tumors. Tumor cell penetration into the blood circulation increased in R-Ras-deficient mice, supporting a role for R-Ras in controlling tumor vessel permeability. Decreased blood perfusion of tumor vessels in R-Ras-deficient mice elevated tumor hypoxia, which, in turn, led to decreased efficacy of radiotherapy in these mice.
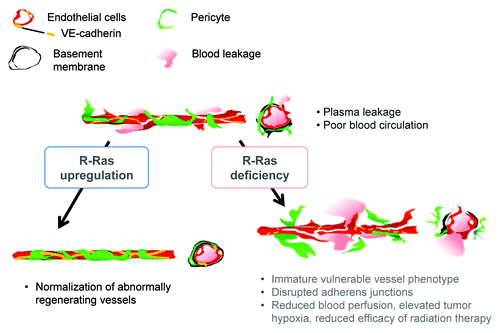
In contrast to R-Ras disruption, R-Ras gain-of-function improved vessel structure and blood perfusion and blocked plasma leakage by enhancing endothelial barrier function and pericyte association with nascent blood vessels.Citation3 The studies of cell type-specific expression of R-Ras revealed that R-Ras in endothelial cells and in pericytes both contribute individually to the vessel regulation.Citation3 Thus, R-Ras promotes normalization of the tumor vasculature (). R-Ras is abundantly expressed in normal blood vessels. In comparison, R-Ras is expressed only at very low levels in most blood vessels in mouse and human tumors, supporting the idea that chronically reduced R-Ras expression in tumor vessels causes the immaturity and abnormality of these vessels.
Figure 1. Schematic summary of the consequences of R-Ras disruption and upregulation in tumor blood vessels. R-Ras is expressed at low levels in the endothelium and pericytes of tumor vessels. The disruption of R-Ras severely impairs structural and functional maturation of tumor vessels. These vessels exhibit the poorly pericyte-supported “vulnerable” immature phenotype. The vessel abnormalities result in extensive blood leakage, reduced blood perfusion and elevated hypoxia within tumors. On the other hand, upregulation of R-Ras signaling enhances pericyte association and stabilizes VE-cadherin-mediated endothelial adherens junctions, leading to improved vessel structure and endothelial barrier function with improved blood perfusion. Thus, R-Ras promotes normalization of pathologically regenerating blood vessels.
Previously, Mazzone et al. reported that haplodeficiency of the oxygen-sensing prolyl hydroxylase domain protein 2 (PHD2) promotes normalization of tumor vasculature by stabilizing endothelial HIF-2α and consequently upregulating expression of soluble VEGFR1 and VE-cadherin in endothelial cells.Citation5 The effect of PHD2 haplodeficiency and HIF-2α stabilization exemplifies the significance of oxygen sensing by endothelial cells for vessel integrity.Citation6 Our data did not support the PHD2/HIF-2-dependent endothelial cell regulation as a mechanism by which R-Ras promotes vascular normalization. In our study, constitutively activated R-Ras did not alter VE-cadherin expression in endothelial cells; rather, it inhibited VE-cadherin internalization induced by VEGF-stimulation, resulting in VE-cadherin accumulation at adherens junctions and stabilization of the endothelial barrier. Furthermore, our previous study of human R-RAS transcriptional regulation did not identify HIF response elements in the R-RAS promoter or in 5′ upstream cis-regulatory sequences.Citation7 This observation suggests that, unlike many hypoxia-induced angiogenesis regulators, R-RAS expression is not controlled by an oxygen-sensing mechanism involving HIFs. There is an additional important difference between the vessel regulations by PHD2/HIF-2 and by R-Ras. PHD2 haplodeficiency does not affect vessel density, area or vessel dilation, while it normalizes the endothelial barrier and stability.Citation5 In contrast, R-Ras normalizes all of these vessel parameters and halts angiogenic sprouting. It is also noteworthy that the chronic low-oxygen environment is a hallmark of solid tumors, and HIF protein levels are elevated in cells in the tumor microenvironment. Nonetheless, vessels generally remain abnormal despite elevated HIF protein levels. Overall, our findings suggest that an alternative R-Ras-dependent activity, distinct from that of an oxygen-sensing mechanism, can promote vascular normalization.
Although R-Ras is closely related to prototypic Ras oncoproteins, such as K- and H-Ras, R-Ras activity results in biological outcomes distinct from those of prototypic Ras proteins. R-Ras inhibits vascular cell proliferation and invasion and promotes vascular quiescence.Citation4 R-Ras-deficient mice show enhanced tumor angiogenesis as well as exaggerated neointimal proliferation of arterial smooth muscle cells in response to arterial injury.Citation4 Despite the significance of R-Ras in vascular regulation, its downstream effectors are not well-understood. PI3 kinase is one of the few known direct downstream targets of R-Ras, and unlike K- and H-Ras, R-Ras does not activate Raf-1 or RalGDS.Citation8 R-Ras does regulate VE-cadherinCitation3 and integrinsCitation9; however, mechanisms underlining these regulations are not well-understood. Identification of R-Ras signaling pathways could advance our understanding of vascular normalization and other important processes during blood vessel regeneration and remodeling.
References
- Carmeliet P, et al. Nat Rev Drug Discov 2011; 10:417 - 27; http://dx.doi.org/10.1038/nrd3455; PMID: 21629292
- Jain RK. Science 2005; 307:58 - 62; http://dx.doi.org/10.1126/science.1104819; PMID: 15637262
- Sawada J, et al. Cancer Cell 2012; 22:235 - 49; http://dx.doi.org/10.1016/j.ccr.2012.06.013; PMID: 22897853
- Komatsu M, et al. Nat Med 2005; 11:1346 - 50; http://dx.doi.org/10.1038/nm1324; PMID: 16286923
- Mazzone M, et al. Cell 2009; 136:839 - 51; http://dx.doi.org/10.1016/j.cell.2009.01.020; PMID: 19217150
- Coulon C, et al. Arterioscler Thromb Vasc Biol 2010; 30:2331 - 6; http://dx.doi.org/10.1161/ATVBAHA.110.214106; PMID: 20966400
- Xu L, et al. J Biol Chem 2009; 284:2706 - 18; http://dx.doi.org/10.1074/jbc.M808184200; PMID: 19011236
- Ehrhardt A, et al. Exp Hematol 2002; 30:1089 - 106; http://dx.doi.org/10.1016/S0301-472X(02)00904-9; PMID: 12384139
- Zhang Z, et al. Cell 1996; 85:61 - 9; http://dx.doi.org/10.1016/S0092-8674(00)81082-X; PMID: 8620538