To repair extensive areas of tissue loss, a common strategy for many adult organisms is to re-awaken long dormant developmental pathways in order to reconstitute the complete structure of original tissues de novo. Zebrafish and amphibian tissue regeneration requires the formation of a mesenchymal progenitor mass (termed “blastema”) that possesses spatial cues to direct tissue reorganization.Citation1 In most mammals, de novo tissue regeneration is typically limited to replacement of specific units, such as hair folliclesCitation2 and intestinal crypts.Citation3 For this to occur, the proper spatial distribution of epithelial stem and differentiated cells must be re-established. To replace epithelial structures, stem cells must first expand into the wound. Next, stem cells must then become distributed into a developing niche that supports cellular differentiation. These epithelial cell behaviors typically require cues from multiple mesenchymal cell types that modulate developmental pathways, including Wnt and TGF-β/BMP pathways. An important goal is to identify such mesenchymal cell types and their function in repair.
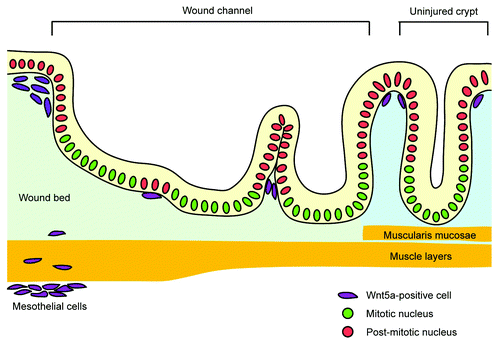
Recently, we reported a new role for Wnt5a-positive mesenchymal cells in mouse colonic wound healing.Citation4 Here we used biopsy forceps to create focal wounds (~200–300 crypts) within the inner surface of the mouse colon. Importantly, this procedure removes epithelial stem cells (localized to crypt bases) from this location. Our lineage-tracing studies showed that existing crypts surrounding the original wound bed (“wound-adjacent crypts”) were the source of new crypts in the wound. These wound-adjacent crypts altered their morphology to create channel-like structures consisting of epithelial cells (“wound channels”) that migrated in the wound (). We initially predicted that canonical Wnt ligands would be increased in the wound bed mesenchyme in order to induce wound channels as canonical Wnt signaling maintains gastrointestinal stem cell activity.Citation5 However, it was the non-canonical ligand, Wnt5a that showed the greatest induction within the wound bed. Wnt5a-positive mesenchymal cells were induced in wounds and were associated with wound channels. Interestingly, Wnt5a-positive cells were preferentially localized near post-mitotic cells within wound channels clefts, suggesting that Wnt5a might induce new crypt formation from wound channels (). We confirmed this hypothesis by injuring the colons of mice with a conditional deletion of Wnt5a. These mice showed a defect in wound channel subdivision into crypts.
Figure 1. Schematic of crypt regeneration during the colonic wound healing. After injury, an adjacent crypt expands into the wound bed and forms a wound channel. In parallel, Wnt5a-positive mesenchymal cells focally suppress proliferation of adjacent epithelial cells in the wound channel and induce its subdivision into crypts.
Non-canonical Wnt signaling in vertebrates is enigmatic because of the multiplicity of reported downstream pathways. While the primary target of canonical Wnt ligands is β-catenin stabilization followed by TCF/LEF-dependent transcription, non-canonical Wnt ligands can potentially target various signaling pathways, including Ca2+, JNK and Rho GTPases. Specific targets may be cell context-dependent.Citation6 Both canonical and non-canonical Wnt ligands share frizzled receptors that are coupled with unique co-receptors, LRP5/6 (canonical) or ROR1/2 (non-canonical). Although the importance of Wnt5a, a representative non-canonical Wnt ligand, has been addressed in mouse development,Citation7 its physiological roles in adult animals remained unknown. To determine the effect of Wnt5a on colonic epithelial cells, we developed a novel organoid culture system for enrichment of colonic epithelial stem cells. In vitro, recombinant Wnt5a inhibited proliferation of colonic stem cells. However, this effect was not due to canonical Wnt suppression. Because the connection between the non-canonical Wnt signaling and cell cycle inhibition was unknown, we first tested if expression of CDK inhibitors were elevated in Wnt5a-treated organoids. p15INK4, a downstream target of TGF-β signaling,Citation8 was robustly induced, generating the hypothesis that Wnt5a either activated or potentiated TGF-β signaling. TGF-β is of high interest here as it is a potent inhibitor of epithelial proliferation.Citation9 In normal colonic crypts, activation of TGF-β signaling is inversely correlated with distribution of proliferating epithelial cells. Its activity is minimal near crypt bases (and stem cells) and is increased in the upper crypt toward the inner surface of the colon. Mature TGF-β ligands bind to the TGF-β receptor complex (type I and type II receptors), which induces phosphorylation of receptor-regulated Smad proteins (R-Smad: Smad2 or Smad3) by the type I receptor. Phosphorylated R-Smad proteins bind to Smad4 and form the Smad transcriptional complex, which induces downstream gene expression, such as p15INK4B (CDKN2B) and PAI-1 (SERPINE1).Citation9 Surprisingly Wnt5a, as well as TGF-β, induced Smad3 phosphorylation and PAI-1 expression in colonic organoids. This effect required the kinase activity of the TGF-β type I receptor, as its specific inhibitor restored growth of Wnt5a-treated organoids. Suppression of ROR2 using shRNA inhibited PAI-1 induction by Wnt5a but did not restore proliferation, suggesting a transcription-independent mechanism of growth suppression. Although CDK inhibitors such as p15INK4B are thought to be the major mediators of TGF-β-induced cell cycle inhibition, a transcription-independent pathway that represses Cdc25 is also proposed.Citation10 We propose that the precise molecular pathways of growth inhibition in colonic stem cells could be determined using our stem cell culture system.
In summary, we identified a novel effect of non-canonical Wnt in colonic epithelial stem cells during injury repair. Both TGF-β and non-canonical Wnt can produce similar outcomes to intestinal epithelial cell proliferation. Our speculative model is that TGF-β signaling potently influences stem cell proliferation. To limit this signaling pathway to a specified area (i.e., the site of a new crypt), Wnt5a induction in mesenchymal cells allows for the precise localization and timing of TGF-β signaling. Future studies will be required to dissect a more precise mechanism by which these two pathways intersect.
References
- Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science 2009; 324:1666 - 9; http://dx.doi.org/10.1126/science.1172687; PMID: 19556498
- Seifert AW, Kiama SG, Seifert MG, Goheen JR, Palmer TM, Maden M. Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature 2012; 489:561 - 5; http://dx.doi.org/10.1038/nature11499; PMID: 23018966
- Seno H, Miyoshi H, Brown SL, Geske MJ, Colonna M, Stappenbeck TS. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci U S A 2009; 106:256 - 61; http://dx.doi.org/10.1073/pnas.0803343106; PMID: 19109436
- Miyoshi H, Ajima R, Luo CT, Yamaguchi TP, Stappenbeck TS. Wnt5a potentiates TGF-β signaling to promote colonic crypt regeneration after tissue injury. Science 2012; 338:108 - 13; http://dx.doi.org/10.1126/science.1223821; PMID: 22956684
- Gregorieff A, Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev 2005; 19:877 - 90; http://dx.doi.org/10.1101/gad.1295405; PMID: 15833914
- Kikuchi A, Yamamoto H, Sato A, Matsumoto S. Wnt5a: its signalling, functions and implication in diseases. Acta Physiol (Oxf) 2012; 204:17 - 33; http://dx.doi.org/10.1111/j.1748-1716.2011.02294.x; PMID: 21518267
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 1999; 126:1211 - 23; PMID: 10021340
- Hannon GJ, Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature 1994; 371:257 - 61; http://dx.doi.org/10.1038/371257a0; PMID: 8078588
- Siegel PM, Massagué J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer 2003; 3:807 - 21; http://dx.doi.org/10.1038/nrc1208; PMID: 14557817
- Iavarone A, Massagué J. Repression of the CDK activator Cdc25A and cell-cycle arrest by cytokine TGF-beta in cells lacking the CDK inhibitor p15. Nature 1997; 387:417 - 22; http://dx.doi.org/10.1038/387417a0; PMID: 9163429