Over half a million patients are diagnosed with Head and Neck Squamous Cell Carcinoma (HNSCC) each year in the world. Most of these patients present with locally advanced tumors, and less than 50% will live for five years after treatment.Citation1 Concurrent platinum-based chemoradiation protocols improve loco-regional control, and have become a standard therapeutic strategy. However, they are associated with acute, high-grade toxicity that includes neutropenia, mucositis, xerostomia and swallowing impairment.
Consumption of alcohol and tobacco smoke is the major risk factor for HNSCC. However, compelling evidence has accumulated over the last decade for infection of head and neck epithelium by high-risk human papillomaviruses (HPVs) as an emerging etiology for HNSCC (about 25% of all HNSCC cases). The prevalence of HPV-related HNSCC is increasing rapidly in North America and North European countries. Patients with HPV-positive tumors constitute a distinct clinical HNSCC subpopulation, who respond better to therapy and have improved relapse-free and overall survival.Citation2
HPV-driven carcinogenesis is mainly a consequence of deregulation of the E6 and E7 viral oncoproteins.Citation3 E6 binds to the p53 tumor suppressor protein and the E6AP cellular E3-ubiquitin ligase (), leading to p53 ubiquitination, proteasomal degradation and impaired function. The functional consequences include loss of p21 mediated control of the G1/S and G2/M checkpoints, reduced DNA-damage repair and cell cycle inhibition; and decreased expression of the pro-apoptotic Bax and Puma factors and consequent diminished cell death. E7 binds to the Retinoblastoma protein (pRb) and induces its degradation (). pRb inhibits cell cycle progression via inhibition of transcription factors of the E2F family. Loss of pRb activates E2F and promotes cell proliferation.
Figure 1. Model representing the role of the E6 and E7 viral oncoproteins in HPV-driven carcinogenesis. (A) The p53 tumor suppressor regulates cell cycle arrest at G1 and G2/M and apoptosis induction via the regulation of its target genes, such as p21, and Puma and Bax respectively. HPV E6-dependent proteasomal degradation of p53 is blocked by Bortezomib. (B) pRb controls the G2/M cell cycle checkpoint via the inhibition of E2F. Binding of pRb to HPV E7 induces its proteasomal degradation, the activation of E2F and stimulation of cell proliferation.
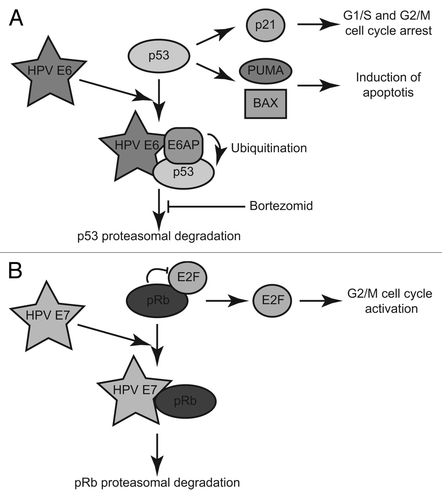
Most HPV-related HNSCC contain wild type TP53,Citation4 and their better response to genotoxic therapies could be due to activation of wild-type p53. Interestingly, inhibition of E6 in HPV-related HNSCC cell lines in culture leads to p53 stabilization and increases cell death.Citation5,Citation6 However, there was no direct evidence that p53 is responsible for this effect. Proof is now provided by Li and Johnson.Citation7 They have confirmed that inhibition of E6 and E7 by siRNA in HPV-positive cell lines leads to p53 stabilization and triggers apoptotic cell death. In addition, they elegantly demonstrate the direct implication of p53 in this induced cell death, by additional inhibition of p53. The authors further observe that Bortezomib, a proteasome-inhibitor (), increases p53 and p21 levels and results in dose-dependent death of HPV-positive cells. This effect is partially impaired by anti-p53 siRNA treatment, showing that p53 is implicated to some extent downstream of Bortezomib. Interestingly, the use of sublethal doses of Bortezomib leads to cell cycle arrest of HPV-positive cell cultures at either G1 or G2/S, to various extends depending on the cell line. The authors provide convincing evidence that this effect is also dependent on both p53 and p21.
The impact of HPV on improving the survival of patients with HNSCC is now clearly established, and there is a debate about de-escalating and/or modulating standard therapies in order to both better manage HPV-related HNSCC and spare patients treatment-related toxicities. In this context, the work by Li and Johnson provides evidence for a direct role of p53 in the increased sensitivity of HPV-positive lesions to genotoxic agents. It also suggests that liberation of p53 from HPV E6 oncroprotein mediated degradation, by inhibition of E6 or stabilization with the proteasome inhibitor bortezomib, could be interesting therapeutic options. Further work is required to assess the efficacy of bortezomib on irradiated HPV-positive and negative cell cultures. A recent phase I clinical trial has demonstrated that the use of bortezomid with concurrent cisplatin-based chemoradiotherapy is well tolerated by HNSCC patients.Citation8 In the light of these new findings, it will be interesting to evaluate tumor response with respect to HPV-status.
References
- Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer 2011; 11:9 - 22; http://dx.doi.org/10.1038/nrc2982; PMID: 21160525
- Kostareli E, Holzinger D, Hess J. New Concepts for Translational Head and Neck Oncology: Lessons from HPV-Related Oropharyngeal Squamous Cell Carcinomas. Front Oncol 2012; 2:36; http://dx.doi.org/10.3389/fonc.2012.00036; PMID: 22655271
- Longworth MS, Laimins LA. Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol Mol Biol Rev 2004; 68:362 - 72; http://dx.doi.org/10.1128/MMBR.68.2.362-372.2004; PMID: 15187189
- Westra WH, Taube JM, Poeta ML, Begum S, Sidransky D, Koch WM. Inverse relationship between human papillomavirus-16 infection and disruptive p53 gene mutations in squamous cell carcinoma of the head and neck. Clin Cancer Res 2008; 14:366 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-07-1402; PMID: 18223210
- Sirianni N, Wang J, Ferris RL. Antiviral activity of Cidofovir on a naturally human papillomavirus-16 infected squamous cell carcinoma of the head and neck (SCCHN) cell line improves radiation sensitivity. Oral Oncol 2005; 41:423 - 8; http://dx.doi.org/10.1016/j.oraloncology.2004.11.003; PMID: 15792615
- Rampias T, Sasaki C, Weinberger P, Psyrri A. E6 and e7 gene silencing and transformed phenotype of human papillomavirus 16-positive oropharyngeal cancer cells. J Natl Cancer Inst 2009; 101:412 - 23; http://dx.doi.org/10.1093/jnci/djp017; PMID: 19276448
- Li C, Johnson DE. Liberation of functional p53 by proteasome inhibition in human papilloma virus-positive head and neck squamous cell carcinoma cells promotes apoptosis and cell cycle arrest. Cell Cycle 2013; 12; In press PMID: 23421999
- Kubicek GJ, Axelrod RS, Machtay M, Ahn PH, Anne PR, Fogh S, et al. Phase I trial using the proteasome inhibitor bortezomib and concurrent chemoradiotherapy for head-and-neck malignancies. Int J Radiat Oncol Biol Phys 2012; 83:1192 - 7; http://dx.doi.org/10.1016/j.ijrobp.2011.09.023; PMID: 22245208