During the process of tumorigenesis, the microenvironments of cancer cells are constantly undergoing changes and remodeling. Such changes and remodeling can lead to shortage of blood supply, reactive immune responses and damage to cellular components (). Any environmental fluctuations leading to deviations from physiological homeostasis are considered as stress to cancer cells. It is well known that strategies taken to modulate stress signaling are critical to tumor development. This also marks a distinction of malignant cells from normal ones. Currently, evolving evidence suggests that microRNAs play key roles in stress response mediation.Citation1 MicroRNAs have been shown to exert diverse functions in cancer cell proliferation, cell cycle progression, invasion and angiogenesis.Citation2-Citation4 Notably, microRNAs regulate cellular metabolism in a cell-specific and context-dependent manner.
Figure 1. By targeting multiple pathways, microRNA networks such as miR-17-associated pathway play a key role in regulating stress response of cancer cells. These stresses may come from chemotherapy treatment, lack of blood supply, cell apoptosis and immune attack. To respond to these stresses, miR-17 could mediate drug resistance, angiogenesis, cell survival and immune evasion.
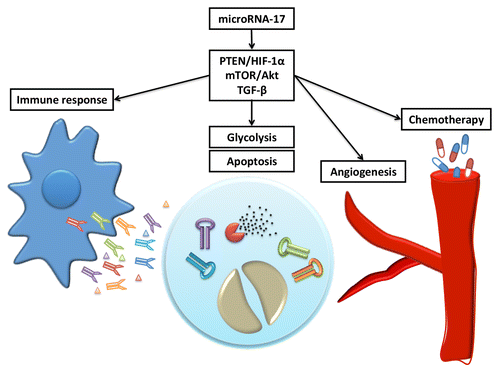
Recent research from our lab has gained insight into this issue, using glioblastoma cells as a model to test the context-dependent functions of microRNA. Glioblastoma is characterized by aggressive angiogenesis and the generation of tumor stem-like cells (TSCs), making it an optimal candidate to test TSC-related phenotypes. Initially, we found that microRNA miR-378 accumulated in glioblastoma U87 cells when deprived of serum in vitro, and miR-378, in turn, contributed to tumor angiogenesis in vivo.Citation5 This led us to further examine alterations to the microRNA network when cancer cells were starved. Under these conditions, we found a group of microRNAs upregulated therein, including miR-17.Citation6 MiR-17 has a controversial role in different cancers: it can function either as an oncomir or as a tumor-suppressor depending on the tumor type. Glioblastoma cells overexpressing miR-17 appeared “highly adaptive” as compared with the other cancerous cells. Under favorable conditions, the proliferative capacity of miR-17-expressing cells decreased. By reducing their metabolic rate, such growth retardation could protect them from serum-starvation. As a result, these cells showed increased survival under serum-free conditions. Moreover, miR-17-expressing cells became more resistant to treatments with cytotoxic reagents, since most chemotherapeutic drugs function by diminishing highly proliferative tumor cells. These effects appeared to be the consequence of miR-17 targeting MDM2 and PTEN. Through the negative regulation of p53, MDM2 acts as an oncogene, and suppression of MDM2 resulted in reduced proliferation. However, PTEN is a tumor suppressor gene that dominates the PTEN/Akt/HIF-1alpha pathway. Downregulating PTEN caused activation of HIF-1alpha, which contributed to tumor survival and angiogenesis. Interestingly, HIF-1alpha expression was only stabilized under stressed conditions and acted as a sensor to detect environmental fluctuation. Activation of HIF-1alpha in response to chemotherapy not only prolonged glioblastoma cell survival, but also accelerated the transformation of TSCs. Tumor stem-like cells have been identified as one of the most important causes of tumor recurrence. It is believed that a subpopulation of cancer cells is capable of preserving their tumorigenicity after cytotoxic chemotherapy. But how these cells remain undamaged after treatment is not readily explained by current theory.
The glioblastoma TSCs show characteristic overexpression of miR-17, which restricts cell growth to an indolent pattern. Interestingly however, these cells were more able to resist drug treatment and generate secondary colonies. Particularly under stressed conditions, TSCs were enriched in glioblastoma cells overexpressing miR-17. These cells showed a greater ability to induce angiogenesis when the nutritional supply was decreased, which may contribute to evasion of traditional chemotherapeutic treatment. Thus, the effects of miR-17 in glioblastoma are 2-fold. First, by shifting the metabolic requirements during periods of tumor growth, these malignant cells can evade traditional chemotherapy regimens. Second, increased angiogenic capacity allows these cancer cells to rapidly regrow through increased tumor vascularization.
The realization that microRNAs play a dual role in glioblastoma cells will provide a new perspective to our understanding of stress responses in cancer. To adapt to fluctuations in environmental stress, microRNA networks can balance signaling by targeting both positive and negative regulators of tumor progression.Citation7 Any changes leading to imbalanced signaling might trigger the responses of microRNAs accordingly. The heterogeneity of cancer lies not only in its genetic diversity, but also in its wide array of modifications at the post-transcriptional level, indeed posing a new challenge in developing cancer therapeutics. For example, traditional therapy may fail to eliminate miR-17-overexpressing cancer cells, which are inherently resistant to current drug treatments. Targeted therapy might also lead to acquired drug resistance if microRNA altered the therapeutic target secondary to survival pressure. Anti-angiogenic therapy is commonly used in treating advanced glioblastoma patients, but drug resistance has been observed frequently. The finding that miR-17 contributes to glioblastoma angiogenesis by over-inducing expression of VEGF provides another possible mechanism of resistance to anti-angiogenic therapy.
Subpopulations of cancer cells with stem cell-like properties have been shown to give rise to secondary tumors following traditional chemotherapy. Understanding the contributions of miRNA to these phenotypes will be valuable. For example, glioblastoma patients with high levels of miR-17 might benefit more from surgical resection, instead of chemotherapy. To address microRNA-induced drug resistance, our lab has developed an anti-miRNA sponge that can efficiently decrease specific microRNA activity in vitro and in vivo.Citation2 The ability of microRNAs to mediate TSC functioning highlights the importance of understanding microRNA networks in cancer progression. Much work needs to be done to uncover the intricacies of miRNA functioning. Understanding the mechanism may provide unprecedented opportunity in targeted cancer therapy.
Acknowledgements
Our studies were supported by grants from Canadian Institutes of Health Research (MOP-102635, MOP-111171) to B.B.Y. who is the recipient of a Career Investigator Award (CI 7418) from the Heart and Stroke Foundation of Ontario. H.L. is a recipient of the Connaught International Student Award.
References
- Leung AK, Sharp PA. MicroRNA functions in stress responses. Mol Cell 2010; 40:205 - 15; http://dx.doi.org/10.1016/j.molcel.2010.09.027; PMID: 20965416
- Yang X, Rutnam ZJ, Jiao C, Wei D, Xie Y, Du J, et al. An anti-let-7 sponge decoys and decays endogenous let-7 functions. Cell Cycle 2012; 11:3097 - 108; http://dx.doi.org/10.4161/cc.21503; PMID: 22871741
- Fang L, Du WW, Yang W, Rutnam ZJ, Peng C, Li H, et al. MiR-93 enhances angiogenesis and metastasis by targeting LATS2. Cell Cycle 2012; 11:4352 - 65; http://dx.doi.org/10.4161/cc.22670; PMID: 23111389
- Siragam V, Rutnam ZJ, Yang W, Fang L, Luo L, Yang X, et al. MicroRNA miR-98 inhibits tumor angiogenesis and invasion by targeting activin receptor-like kinase-4 and matrix metalloproteinase-11. Oncotarget 2012; 3:1370 - 85; PMID: 23211491
- Lee DY, Deng Z, Wang CH, Yang BB. MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc Natl Acad Sci U S A 2007; 104:20350 - 5; http://dx.doi.org/10.1073/pnas.0706901104; PMID: 18077375
- Li H, Yang BB. Stress response of glioblastoma cells mediated by miR-17-5p targeting PTEN and the passenger strand miR-17-3p targeting MDM2. Oncotarget 2012; 3:1653 - 68; PMID: 23391506
- Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell 2012; 148:1172 - 87; http://dx.doi.org/10.1016/j.cell.2012.02.005; PMID: 22424228