Abstract
MYC (c-Myc) deregulation has been frequently associated with aggressive lymphomas and adverse clinical outcome in B-cell malignancies. MYC has been implicated in controlling the expression of miRNAs, and MYC-regulated miRNAs affect virtually all aspects of the hallmarks of MYC-driven lymphomas. Increasing evidence has indicated that there is significant cross-talk between MYC and miRNAs, with MYC regulating expression of a number of miRNAs, resulting in widespread repression of miRNA and, at the same time, MYC being subjected to regulation by miRNAs, leading to sustained MYC activity and the corresponding MYC downstream pathways. Thus, these combined effects of MYC overexpression and downregulation of miRNAs play a central regulatory role in the MYC oncogenic pathways and MYC-driven lymphomagenesis. Here, we provide biological insight on the function of MYC-regulated miRNAs, the mechanisms of MYC-induced miRNA repression, and the complicated feedback circuitry underlying lymphoma progression, as well as potential therapeutic targets in aggressive B-cell lymphomas.
Introduction
MYC is a basic helix–loop–helix leucine zipper transcription factor that coordinates the diverse transcriptional programs necessary for cell growth, proliferation, invasion, expansion, and angiogenesis.Citation1,Citation2MYC’s highly pleiotropic effects are mirrored by thousands of MYC target genes with roles in virtually every aspect of cell biology and oncology.Citation3MYC is one of the most commonly overexpressed oncogene in cancer and one of the most robust and significant prognostic markers for B-cell lymphomas. MYC dysregulation has been implicated in the aggressive transformation of B-cell lymphomas.Citation4 Although MYC has been described as a defining feature and the driving oncogene for Burkitt lymphoma, MYC has also been recognized in other non-Hodgkin B-cell lymphomas. MYC has been detected in 9–14% of diffuse large B-cell lymphomas, associated with an adverse prognosis as a result of chemoresistance and shortened survival.Citation5,Citation6 In mantle cell lymphoma (MCL), increased expression of MYC has been found to be associated with poor prognosis and MCL aggressiveness.Citation7-Citation9 MYC overexpression has been implicated in high-grade large cell transformation in follicular and marginal zone cell lymphomas, supporting the features of MYC in sustaining aggressive transformation of lymphomas.Citation10
However, the underlying mechanisms for MYC action remain elusive in these lymphomas. The direct MYC-induced transcriptional changes that promote cell transformation are still unclear. Important insights into the molecular pathology of MYC-driven B-cell lymphomas could be gained through a better understanding of which targets are responsible for the biological consequences of MYC suppression or induction. It is increasingly clear that the MYC-targeted gene network also includes non-protein coding targets. Among the latter, microRNAs (miRNAs) have attracted the most attention as important regulators of MYC-driven lymphomagenesis. miRNAs are 20- to 22-nucleotide non-coding RNAs found in plants and animals that inhibit gene expression by targeting mRNAs to degradation or inhibiting translation of mRNAs.Citation11 The human genome encodes thousands of miRNAs, which regulate a large fraction of the human transcriptome. MYC has been recently implicated in controlling the expression of a host of miRNAs.Citation12-Citation14 The predominant consequence of MYC activation is widespread repression of miRNA expression.Citation12-Citation14 Here, we will summarize the role of MYC-regulated miRNAs, especially MYC-repressed miRNAs in MYC-mediated oncogenic processes, and discuss the molecular mechanisms of MYC-induced miRNA repression. Finally, we will exploit the MYC-miRNA circuitry as a mechanism to sustain MYC hyperactivity and as a potential therapeutic target for MYC-driven B-cell malignancies.
MYC-Regulated miRNAs Are Associated with the Hallmarks of B-Cell Lymphomas
miRNAs have been shown to be associated with many of the classical hallmarks of cancer, including proliferation, differentiation, angiogenesis, and apoptosis. With their widespread range of influence on biological pathways and implications as either oncogenes or tumor suppressor genes, their dysregulation justifies their significant role in tumorigenesis leading to lymphoma.
The identification and role of MYC-regulated miRNAs were initially established through chromatin immunoprecipitation and miRNA expression array by using MYC-inducible cell lines, human and mouse models of B-cell lymphoma.Citation13,Citation14 Unexpectedly, the predominant consequence of activation of MYC is widespread repression of miRNA expression.Citation14 Moreover, enforced expression of repressed miRNAs diminishes the tumorigenic potential of lymphoma cells in vitro and in vivo, supporting that MYC-repressed miRNAs function as tumor suppressor genes. Indeed, miRNA transcripts repressed by MYC include several with potent tumor suppressor activity, such as miR-15a/16-1, miR-34a, and let-7 family members. Given the ability of a single miRNA to regulate hundreds of targets, it comes as no surprise that MYC can exert pleiotropic effects and cellular functions through reprogramming of miRNA expression. Here, we highlight some recent advances on how miRNA regulation has been integrated into the MYC oncogenic program in the regulation of hallmarks of B-cell malignancies (see ).
Figure 1. MYC-regulated miRNAs and the hallmarks of B-cell lymphomas. MYC and miRNAs form forward-feedback (double-negative) regulatory loops contributing to sustained MYC activation, miRNA downregulation, and subsequent dramatic deregulation of the hallmarks of lymphoma. “↑”, upregulation; “↓”, repression by MYC. MYC utilizes one or more of the above mechanisms in combination to exert its oncogenic functions.
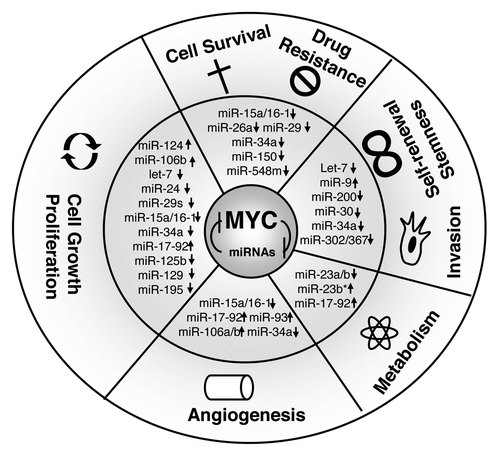
Cell growth and proliferation
As expected, both induction and repression of specific miRNAs by MYC broadly impact MYC-mediated phenotypes, facilitating cell cycle entry and progression by controlling all levels of the cell cycle-regulatory machinery. The influence of these miRNAs on the cell cycle are mediated through inhibition of cell cycle inhibitors such as INK4 or Cip/Kip families by MYC-induced oncogenic miRNAs or through cell cycle-positive regulators, such as cyclins or cyclin kinases, via MYC-repressed miRNAs. For example, the miR-15a/16-1 cluster directly regulates cell cycle progression and proliferation by controlling the G1 checkpoint proteins. Overexpression of miR-16 leads to induction of G0/G1 arrest in tumor cells by suppressing the identified miR-15a/16-1 targets, including CDK1, CDK2, CDK6, as well as cyclins D and E.Citation15 Thus, loss or repression of miR-15a/16-1, often observed in B-cell lymphomas, resulted in induction of these cell cycle-positive regulators and led to lymphoma cell growth and proliferation. The cell cycle kinase binds cyclins in early G1 phase and participates in the sequential phosphorylation of RB1 by CDK4/6 and CDK2 to repress RB inhibition of E2F, subsequently promoting G1-to-S-phase progression.Citation15-Citation18 CDK4 or CDK6 mRNAs are also regulated by other MYC-regulated miRNAs such as miR-24, miR-29, miR-34a, miR-124, miR-125b, miR-195, and let-7 family members.Citation19,Citation20 In addition, cyclin D levels are downregulated by let-7, miR-15 family, miR-17, miR-19a, miR-20a, and miR-34.Citation21 Moreover, miR-16 and miR-34a downregulate cyclin E to regulate cell growth.Citation18 Thus, when MYC is activated in aggressive B-cell lymphomas, these tumor suppressor miRNAs are inactivated by MYC, resulting in induction of these cell cycle-positive regulators and leading to cell proliferation and growth. On the other hand, MYC-activated miRNAs are also involved in cell proliferation and cell cycle progression. p21Cip1, a p53 target and CDK inhibitor, and pRB (retinoblastoma) are direct targets of miR-17-92 and miR-106b.Citation22,Citation23 By targeting this cell cycle inhibitor, MYC-induced activation of miR-17 and miR-106b promotes cell cycle progression.
Cell survival and drug resistance
A number of MYC-regulated miRNAs are involved in the regulation of lymphoma cell survival and drug resistance. Repression of miRNAs by MYC contributes to cellular survival by activation of anti-apoptotic proteins. For example, miR-15a/16-1, miR-26a, miR-29, miR-34a, and miR-150, which are repressed by MYC, can each activate several survival signaling pathways in B-cell lymphomas. Expression of these miRNAs inhibits cell proliferation, promotes apoptosis of lymphoma cells, and suppresses tumorigenicity both in vitro and in vivo.
The miR-15a/16-1 cluster directly downregulates the anti-apoptotic protein Bcl-2, Mcl-1, CCND1, and WNT3A. Downregulation of these miRNAs has been reported in B-cell malignancies.Citation24 MiR-34a is a direct transcriptional target of p53 and contributes to p53-dependent apoptosis.Citation25,Citation26 Recently, Craig et al. reported that, of the MYC-repressed miRNAs that are downregulated in malignant lymphoma, miR-34a showed the strongest antiproliferative properties when overexpressed, and loss of miR-34a resulted in high proliferation in diffuse large B-cell lymphoma cells. This study further attributes miR-34a’s tumor-suppressive effects to deregulation of its target Foxp1. Our studies revealed that the miR-29 family (miR-29a-c) is inversely correlated with MYC expression and regulates cell growth and survival by targeting CDK6, IGF-1R, TCL-1, PI3K, and MCL1.Citation20,Citation27,Citation28 We and others further demonstrated that miR-26a and miR-548 are repressed by MYC, contributing to lymphoma cell survival through silencing of EZH2 and HDAC6, respectively.Citation20,Citation27 Collectively, these observations highlight the broad impact of MYC-mediated miRNA reprogramming on cellular survival and proliferation pathways.
Angiogenesis and metabolism
In aggressive B-cell lymphomas, as in several other cancers, neo-angiogenesis and production of proangiogenic factors such as vascular endothelial growth factor (VEGF) play a central role in lymphoma progression. MYC may conceivably act as a VEGF transcriptional factor, promoting angiogenesis and vasculogenesis by upregulating the expression of proangiogenic factors.Citation29 Consistent with these activities, MYC deactivation has been shown to contribute to the collapse of tumor.Citation30 The mechanism through which MYC regulates the VEGF axis has not yet been clearly elucidated. Several lines of evidence revealed that MYC controls VEGF by regulating a broad range of miRNAs. VEGF translation is regulated by at least 8 miRNAs, including miR-15a, miR-16, miR-17, miR-20a, miR-34a, miR-93, miR-106a, miR-106b, and all of these miRNAs are under the control of MYC.Citation31 MYC-driven angiogenesis can also be mediated by miRNAs through repression of antiangiogenic factors such as TSP1 (thrombospondin-1) and CTGF (connective tissue growth factor). miR-17-92 family members directly target the transcripts that encode TSP1 and CTGF, respectively, thereby reducing expression of these antiangiogenic proteins, and thus increasing angiogenesis.Citation32
In addition, MYC activates the expression of genes to generate bioenergetic substrates for rapid cell growth and high metabolic activity in aggressive MYC-driven B-cell lymphomas. Both in vitro and in vivo models have provided substantial evidence that MYC induces many genes involved in ribosome biogenesis as well as genes involved in glucose and glutamine metabolism to accommodate to growing lymphoma cells.Citation33 Mitochondrial glutaminase was among the proteins identified that are regulated by MYC, specifically through direct suppression of miR-23a and miR-23b. Suppression of these miRNAs triggers an addiction to glutamine, which is required for bioenergetics, nucleotide biosynthesis, and redox homeostasis in cancer cells. Using MYC-inducible human Burkitt lymphoma model P493-6 cells, it was shown that MYC suppressed proline oxidase expression primarily and regulated proline metabolism through upregulation of miR-23b*.Citation34 Furthermore, the induction of the miR-17-92 cluster by MYC attenuates E2F1 protein expression, such that interruption of this regulatory loop results in DNA replication stress. The miR-17-92 cluster is also involved in glycolysis via potentiating signaling through the PI3K-AKT pathway. It is well established that the PI3K signaling route is engaged in glucose and fatty acid metabolism through multiple mechanisms by increasing glucose transporter surface expression and enhancing glycolytic enzyme.Citation35 AKT also activates ATP citrate lyase to promote glucose-dependent fatty acid synthesis and tumor growth in vivo.Citation36 Taken together, in aggressive B-cell lymphoma, activation of MYC orchestrates the expression of genes and miRNAs to meet the bioenergetic and biosynthetic demands of increased cell growth and proliferation.
Self-renewal, stemness, and invasion
Another biological setting in which MYC and miRNA regulation may converge is stem cell self-renewal and invasion. Genetic studies in mice and in embryonic stem (ES) cells reveled that MYC has been shown to be required for the maintenance of self-renewal.Citation37 Furthermore, MYC family genes, together with OCT4, KLF4, and SOX2, act to reprogram differentiated cells into induced pluripotent stem (iPS) cells with ES cell properties, suggesting that MYC is a driver of pluripotency.Citation38,Citation39 Thus, MYC-targeted miRNAs and targeting-MYC miRNAs are involved in lymphoma self-renewal and invasion. Among the miRNAs, best correlating with a stem cell property (stemness) and self-renewal is miR-34. miR-34 family members downregulate the expression of stemness factors, such as SNAIL1, BMI1, CD44, and CD133. The functional relevance of these miRNAs in cancer cells was further validated by demonstrating that miR-34 is necessary for p53-mediated inhibition of important tumor cell properties, including migration and invasion.Citation40 In lymphomas, loss of miR-34a promoted B-cell accumulation and high-grade transformation, and Foxp1 was a direct target of miR-34a in a 3′-untranslated region (UTR)-dependent fashion.Citation41,Citation42 Another miRNA family with a critical role in tumor self-renewal and invasion is let-7. The let-7 target genes, HMGA2, IMP-1, and LIN28B, as well as Ras and MYC have all been reported to be important in acquisition of cancer cell stemness.Citation43 Indeed, let-7 has been associated with genesis and maintenance of the lymphoma aggressive phenotype in Burkitt lymphoma cells and B-cell differentiation.Citation44,Citation45 Similar to let-7, miR-30 is reduced by MYC, and its target proteins Ubc9 (ubiquitin-conjugating enzyme 9) and ITGB3 (integrin beta3) are markedly upregulated in breast cancer stem cell and associated with tumor progression, metastasis, and post-treatment relapse. Our recent study revealed that miR-30 family members are involved in B-cell differentiation.Citation45
miR-200 members, another MYC-targeted miRNA family, which directly target the self-renewal regulator, polycomb ring finger oncogene BMI1, have been found to be highly downregulated in human breast stem cell as compared with non-tumorigenic cancer cells.Citation46,Citation47 Inhibition of miR-200b upregulates the expression of SUZ12, a subunit of a PRC2 (polycomb repressor complex 2), and is also required for mammosphere growth by repressing E-cadherin expression and increasing cell migration and growth.Citation48 Further, miR-200s promotes the self-renewal and stemness through repressing the expression of ZEB1, ZEB2 (zinc finger E-box binding homeobox 1 and 2). ZEB1 has been described as able to directly suppress the transcription of miR-200 family members via a negative feedback loop, and is thought to regulate epithelial–mesenchymal transition (EMT) and to promote the invasion of cancer cells.Citation49 Similarly, miR-302/367 cluster is regulated by the stem cell transcription factors OCT4 and SOX2, as well as MYC. Overexpression of miR-302 alone is enough to reprogram various somatic cells into induced pluripotent stem cells.Citation50 MYC also induces expression of miR-9.Citation51 Through targeting E-cadherin, miR-9 promotes tumor cell migration and invasion, B-lymphocyte differentiation, and MYC-driven lymphomagenesis.Citation45,Citation52 In conclusion, miRNAs are emerging as important markers and key regulators of cancer stem cell (CSC) by suppressing CSC-specific genes and activities, including self-renewal, invasion, and lymphoma aggressive transformation.
Mechanisms of miRNA Repression by MYC
Although the mechanisms by which MYC activates gene transcription are relatively well known, less is known about how MYC represses transcription of target genes, including miRNAs. While genetic alteration of miRNA loci can be one cause of miRNA downregulation, it is likely that MYC hyperactivity also contributes to widespread downregulation of miRNA expression through transcriptional and post-transcritional regulation. The mechanisms through which MYC represses miRNAs are therefore of particular significance, since reversing these effects could have important therapeutic implications. Our study revealed that MYC acts as a repressor of miR-15a/16-1 by recruiting HDAC3.Citation53 To investigate the role of histone acetylation and HDAC in MYC-induced miRNA repression, we performed miRNA expression profiling in lymphoma cells after pan-HDAC inhibitor, suberoylanilide hydroxamic acid (SAHA) treatment. We observed that SAHA indeed induced expression of a set of MYC-regulated miRNAs, including miR-29c, miR-26a, miR-30, and miR-15a/16-1. These findings suggest that histone deacetylation is involved in MYC-mediated transcriptional repression. Moreover, we recently demonstrated that MYC, HDAC3, and PRC2 form a repressive complex tethered to miR-29 and miR-26a promoter elements to epigenetically repress transcription of these miRNAs in MYC-expressing lymphoma cells.Citation20 These results indicated that EZH2-mediated histone methylation is involved in MYC regulation of miRNA expression. This is further supported by our miRNA array study showing that EZH2 inhibition induced expression of a number of MYC-regulated miRNA such as miR-101, miR-200, miR-494, and miR-548.Citation20,Citation54 Collectively, these findings reveal that epigenetic histone acetylation and/or methylation are novel mechanisms for MYC-mediated miRNA transcriptional repression ().
Figure 2. Potential mechanisms of miRNA repression by MYC. (A) MYC recruits HDAC complex to miRNA promoter (of miR-15a/16-1) to induce histone tail deactylation, compact chromatin, and lead to miRNA transcription suppression. (B) PRC2 is tethered to miRNA promoter (of miR-26a, miR-29 and miR-548 min) with HDAC3 by MYC to methylate and deacetylate histone tail and subsequently inhibits miRNA transcription. (C) DNMT3 is recruited to miRNA promoter (of miR-34a and miR-146a) by MYC to methylate DNA and lead to repression of miRNA transcription. (D) MYC induces Lin-28B expression through direct association with the Lin-28 promoter, and Lin-28 proteins act as negative regulators of miRNAs (let-7 and miRNA-150) maturation and biogenesis.
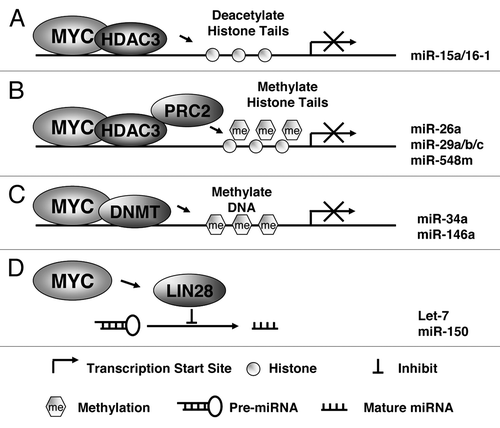
One of the most common causes of the loss of tumor-suppressor miRNAs in B-cell lymphomas is the silencing of their primary transcripts by CpG island promoter hypermethylation. One line of evidence suggests that MYC recruits the DNA methyltransferase DNMT3a to the promoters of its negatively regulated target genes, an example of which is p21. DNA methylation-based regulation of miRNAs has also been recently described.Citation55 To investigate whether miR-34a promoter methylation contributes to its repression in aggressive gastric lymphoma, Craig et al. performed methylation-specific PCR analyses of a CpG island that surrounds the transcriptional start site of the miR-34a.Citation42 Primary miRNA-34a exhibited promoter hypermethylation in high-grade large B-cell lymphoma, indicating that epigenetic silencing through DNA methylation may contribute to miR-34a repression and constitute another mechanism for MYC-induced miRNA repression.Citation42 Promoter methylation of miR-146a, another MYC-downregulated miRNA has been described in primary aggressive NK/T-cell lymphomas and is associated with low expression level and poor prognosis.Citation56 Therefore, methylation may occur in promoter-associated CG islands of multiple miRNAs regulating MYC signaling pathways and functions (). Another layer of complexity for MYC miRNA regulation is miRNA processing (). Once transcribed, miRNAs are processed and exported from the nucleus to the cytoplasm. Alterations in the processing machinery can also lead to deregulation of functional miRNAs. Chang et al. revealed that MYC-mediated transactivation of the RNA-binding protein Lin-28B is necessary for MYC’s ability to post-transcriptionally repress let-7 family members.Citation57 The study showed that MYC induces Lin-28B expression through direct association with the Lin-28 promoter, and that Lin-28 proteins act as negative regulators of let-7 maturation and biogenesis. Lin-28 regulates the expression of let-7 by binding to the precursors and blocking their maturation. More recently, Jiang et al. showed that the maturation of another MYC-repressed miRNA, miR-150, is also regulated by MYC-LIN28 axis, and mixed lineage leukemia (MLL) fusion proteins negatively regulate production of miR-150 by blocking miR-150 precursors from being processed to mature miRNAs through MYC/LIN28 functional axis.Citation58 In summary, these data uncover an orchestration of transcriptional and posttranscriptional mechanisms in MYC-mediated reprogramming of miRNA expression (). Epigenetic silencing through DNA methylation or histone acetylation and/or methylation and disruption of miRNA biogenesis contribute to MYC-induced miRNA repression, and MYC utilizes one or more of the above mechanisms in combination to exert its oncogenic functions.
In addition, MYC also upregulates expression of a set of oncogenic miRNAs. Perhaps the most well-studied oncogenic miRNA induced by MYC is the miR-17-92 cluster, also known as Oncomir-1. This miRNA cluster is located within the noncoding gene C13orf25 at 13q31.3, which is frequently amplified in B-cell lymphomas and overexpressed in a variety of other tumors.Citation59 Unlike most protein coding genes, miR-17-92 is a polycistronic miRNA cluster that contains multiple miRNA components, each of which has a function to regulate hundreds of target mRNAs. Transgenic expression of this cluster in mice leads to a lymphoproliferative disorder,Citation60 while its genetic ablation impairs normal B-cell development.Citation61 Six mature miRNAs, miR-17, miR-18a, miR-19a, miR-20a, miR-19b-1, and miR-92a-1, are encoded in this cluster. When overexpressed in the lymphoid compartment, miR-17-92 cluster-derived miRNAs cooperate with MYC in inducing lymphomas in the Eµ-myc mouse lymphoma model. Recently, miR-19 was identified as the key oncogenic component of the cluster in this model. In addition, miR-18 was also shown to have some oncogenic potential. The tumor suppressor PTEN and the proapoptotic protein Bim have emerged as important targets repressed by oncomir-1-derived miRNAs in hematopoietic system.Citation59 Hence, the miRNAs in the miR-17-92 cluster regulate multiple functions involved in lymphomagenesis. Ectopic expression of miR-17-92 cooperates with the MYC oncogene in a mouse model of B-cell lymphomas.Citation62 The functional interplay between miR-17-92 and MYC is further supported by the finding that MYC itself is a potent and direct transcriptional activator of miR-17-92,Citation13 thus supporting that miR-17-92 may contribute to the oncogenic properties of MYC. The exact molecular basis underlying miRNA-mediated gene induction is not entirely clear. Our CHIP analysis revealed, in contrast to MYC-repressed miRNAs, much stronger MYC binding and weaker or no HDAC3 or/and EZH2 bindings to the E-box regions of miR-17-92 cluster gene promoter. The different binding complex of MYC and epigenetic modifiers (HDAC and EZH2) is likely attributed to E-box context of different miRNAs and dictates the transcriptional activation or repression function of MYC on miRNAs expression.
MYC Regulation by miRNAs and MYC–miRNA Circuitry as a Mechanism of Sustaining MYC Activity
The interaction between MYC and miRNAs is mutual, as MYC itself is targeted by miRNAs. When interactions between downregulated miRNAs and overexpressed target protein-coding genes were mapped in murine and human lymphomas, MYC was identified as the upregulated gene with the highest interaction with downregulated miRNAs.Citation19 The functionally best-characterized MYC-targeting miRNA is miR-34a, which is located at chromosome 1p36, a region frequently deleted in MYC-associated lymphomas.Citation14 Overexpression of miR-34a in lymphoma cell lines decreased MYC levels, inhibited proliferation, and induced apoptosis.Citation14,Citation63 The let-7 tumor suppressor miRNA, another MYC-repressed miRNA, is also able to downregulate MYC, reverting MYC-induced growth in Burkitt lymphoma cells.Citation44 In addition to miR-34a and let-7, we revealed that miR-135a, miR-186, miR-494, miR-200c, miR-374a/b, miR-101, and miR-548 target MYC. To further validate that these miRNAs target the MYC 3′-UTR directly, we cloned the full-length of MYC 3′-UTR and constructed a luciferase reporter plasmid (p-miR-MYC-3′-UTR-WT). Overexpression with each of the aforementioned pre-miRNAs reduced the luciferase activity and diminished MYC levels, which reduced proliferation and clonogenic growth.Citation20,Citation54 On the other hand, miRNAs regulate MYC indirectly through targeting other MYC-regulatory proteins or miRNAs. The miRNA target proteins regulate MYC expression at transcriptional and posttranscriptional levels. For example, the miR-26a-regulated PRC2 protein, EZH2, modulates MYC expression through EZH2-mediated miR-494 expressions.Citation20,Citation27 We and others have also shown that a combination of MYC-targeting miRNAs has a stronger effect in MYC downregulation than individual miRNAs. Given widespread miRNA repression, including a panel of miRNAs that target MYC in aggressive lymphomas, it is predicted that downregulation of these miRNAs coordinately contribute to MYC activation and induction of MYC downstream oncogenic pathways, leading to lymphoma aggressive progression.
On another note, MYC is present at very low levels in normal cells, both the short-lived MYC protein and its equally short-lived mRNA, indicating that MYC levels are tightly regulated by transcriptional and posttranscriptional mechanisms. However, constitutive MYC activation has been reported in aggressive lymphomas.Citation4 A regulatory mechanism that enhances the strength and prolongs the duration of MYC activity is required for these lymphomas. Indeed, accumulating evidence has shown that many of these MYC-targeting (direct or indirect) miRNAs are silenced by MYC through binding to E-boxes in the promoter regions of these target genes, thus generating forward-feedback (double-negative) regulatory loops between MYC and miRNAs in aggressive B-cell lymphomas (). In contrast to low MYC activation and high expression of MYC-repressed miRNAs in normal and reactive B lymphocytes, in aggressive MYC-associated lymphomas, lymphoma cells acquire diverse genetic or epigenetic alterations that result in MYC overexpresssion. Amplified and overexpressed MYC leads to low levels of these miRNAs, with the negative feedback loops for regulating MYC being abrogated. Thus, MYC-miRNA circuitry through autocrine/paracrine loops contributes to sustained MYC activation and subsequent dramatic deregulation of the cell cycle, protein translation, and metabolism among other cellular processes for lymphomas aggressive progression.
Summary and Perspectives
MYC is a potent oncogene initially identified as the hallmark and driving force in Burkitt lymphoma. Increasing evidence has supported that MYC gene alterations, which have been identified in other mature B-cell neoplasms, are usually associated with aggressive clinical behavior. The advent of functional and structural genomics with advances in immunology analyses and new animal models has greatly accelerated our understanding of oncogenic mechanisms in these MYC-associated lymphomas. The current findings of widespread downregulation of the miRNA transcriptome in aggressive lymphomas clearly indicate a central role of miRNA deregulation in lymphoma initiation and progression. The identification of the MYC-repressed tumor-suppressor miRNAs underlies the molecular mechanism of MYC-induced lymphomagenesis.
MYC has the ability to activate genes that increase the malignancy of the tumor and at the same time has the ability to repress genes such as tumor suppressors. Although the transcription activator mechanisms of MYC are relatively well known, few studies have been conducted to explain how MYC can exert its transcription repression function. One interesting aspect of that emerges from our own studies, showing that MYC can directly interact with many chromatin components, particularly HDAC3 and PRC2 proteins (EZH2, SUZ12), as a novel, genuine, and critical mechanism of MYC-mediated transcriptional repression.Citation20,Citation53 Thus, our findings provide a rational to redirect therapeutic effort by reactivating these MYC-repressed tumor suppressor miRNAs through inhibition of HDAC and/or EZH2. Indeed, we demonstrated that the combination of HDAC and EZH2 inhibitors (vorinostat and DZNep) induced miR-29 gene expression, resulting in the synergistic reduction of oncogenic protein levels of CDK6 and IGF-1R and subsequent inhibition of cell survival and lymphoma formation in vitro and in vivo. Further, the identification of the reciprocal MYC-miRNA feedback circuits added another layer of complexity to the molecular basis of sustaining MYC oncogenic signal and driving lymphoma aggressive progression.Citation20,Citation54 Thus, disruption of the MYC-miRNA loop to suppress aggressive B-cell lymphoma survival and growth can be a novel promising therapeutic approach. Indeed, recently, using a novel small-molecule BET bromodomain inhibitor, JQ1, and the EZH2 inhibitor, DZNep, we demonstrated that combined treatment of JQ1 and DZNep cooperatively disrupted MYC-miRNA, resulting in a greater MYC reduction, restoration of tumor suppressor miRNAs such as miR-26a, and a synergistic suppression of lymphoma growth and clonogenicity in aggressive lymphoma cells.Citation27 Taken together, it is now becoming increasingly evident that interplay between MYC and miRNAs plays a crucial role in aggressive lymphomas. The control of MYC-miRNA interaction is therefore a therapeutic target for control of MYC and MYC downstream pathways and lymphoma therapy. From this perspective, a deep understanding of the nature of the genetic and epigenetic MYC regulation mechanisms and continued efforts to unravel the specific contribution of miRNA deregulation will be needed for improved therapy of aggressive lymphomas.
| Abbreviations: | ||
| MCL | = | mantle cell lymphoma |
Disclosure of Potential Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by grants from the National Cancer Institutes (R01 CA137123, to JT), Maher Fund (to JT), Lymphoma Research Foundation (to JT), National Functional Genomics Center Programmatic Research grand and American Hematology Society Bridge Grant (to JT).
References
- Vita M, Henriksson M. The Myc oncoprotein as a therapeutic target for human cancer. Semin Cancer Biol 2006; 16:318 - 30; http://dx.doi.org/10.1016/j.semcancer.2006.07.015; PMID: 16934487
- Eilers M, Eisenman RN. Myc’s broad reach. Genes Dev 2008; 22:2755 - 66; http://dx.doi.org/10.1101/gad.1712408; PMID: 18923074
- Dang CV. MYC on the path to cancer. Cell 2012; 149:22 - 35; http://dx.doi.org/10.1016/j.cell.2012.03.003; PMID: 22464321
- Ott G, Rosenwald A, Campo E. Understanding MYC-driven aggressive B-cell lymphomas: pathogenesis and classification. Blood 2013; 122:3884 - 91; http://dx.doi.org/10.1182/blood-2013-05-498329; PMID: 24009228
- Savage KJ, Johnson NA, Ben-Neriah S, Connors JM, Sehn LH, Farinha P, Horsman DE, Gascoyne RD. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood 2009; 114:3533 - 7; http://dx.doi.org/10.1182/blood-2009-05-220095; PMID: 19704118
- Valera A, López-Guillermo A, Cardesa-Salzmann T, Climent F, González-Barca E, Mercadal S, Espinosa I, Novelli S, Briones J, Mate JL, et al, Grup per l’Estudi dels Limfomes de Catalunya i Balears (GELCAB). MYC protein expression and genetic alterations have prognostic impact in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Haematologica 2013; 98:1554 - 62; http://dx.doi.org/10.3324/haematol.2013.086173; PMID: 23716551
- Hernández L, Hernández S, Beà S, Pinyol M, Ferrer A, Bosch F, Nadal A, Fernández PL, Palacín A, Montserrat E, et al. c-myc mRNA expression and genomic alterations in mantle cell lymphomas and other nodal non-Hodgkin’s lymphomas. Leukemia 1999; 13:2087 - 93; http://dx.doi.org/10.1038/sj.leu.2401599; PMID: 10602433
- Nagy B, Lundán T, Larramendy ML, Aalto Y, Zhu Y, Niini T, Edgren H, Ferrer A, Vilpo J, Elonen E, et al. Abnormal expression of apoptosis-related genes in haematological malignancies: overexpression of MYC is poor prognostic sign in mantle cell lymphoma. Br J Haematol 2003; 120:434 - 41; http://dx.doi.org/10.1046/j.1365-2141.2003.04121.x; PMID: 12580957
- Hartmann E, Fernàndez V, Moreno V, Valls J, Hernández L, Bosch F, Abrisqueta P, Klapper W, Dreyling M, Hoster E, et al. Five-gene model to predict survival in mantle-cell lymphoma using frozen or formalin-fixed, paraffin-embedded tissue. J Clin Oncol 2008; 26:4966 - 72; http://dx.doi.org/10.1200/JCO.2007.12.0410; PMID: 18606985
- Slack GW, Gascoyne RD. MYC and aggressive B-cell lymphomas. Adv Anat Pathol 2011; 18:219 - 28; http://dx.doi.org/10.1097/PAP.0b013e3182169948; PMID: 21490439
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer 2006; 6:857 - 66; http://dx.doi.org/10.1038/nrc1997; PMID: 17060945
- Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 2009; 458:762 - 5; http://dx.doi.org/10.1038/nature07823; PMID: 19219026
- O’Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature 2005; 435:839 - 43; http://dx.doi.org/10.1038/nature03677; PMID: 15944709
- Chang TC, Yu D, Lee YS, Wentzel EA, Arking DE, West KM, Dang CV, Thomas-Tikhonenko A, Mendell JT. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet 2008; 40:43 - 50; http://dx.doi.org/10.1038/ng.2007.30; PMID: 18066065
- Linsley PS, Schelter J, Burchard J, Kibukawa M, Martin MM, Bartz SR, Johnson JM, Cummins JM, Raymond CK, Dai H, et al. Transcripts targeted by the microRNA-16 family cooperatively regulate cell cycle progression. Mol Cell Biol 2007; 27:2240 - 52; http://dx.doi.org/10.1128/MCB.02005-06; PMID: 17242205
- Liu Q, Fu H, Sun F, Zhang H, Tie Y, Zhu J, Xing R, Sun Z, Zheng X. miR-16 family induces cell cycle arrest by regulating multiple cell cycle genes. Nucleic Acids Res 2008; 36:5391 - 404; http://dx.doi.org/10.1093/nar/gkn522; PMID: 18701644
- Takeshita F, Patrawala L, Osaki M, Takahashi RU, Yamamoto Y, Kosaka N, Kawamata M, Kelnar K, Bader AG, Brown D, et al. Systemic delivery of synthetic microRNA-16 inhibits the growth of metastatic prostate tumors via downregulation of multiple cell-cycle genes. Mol Ther 2010; 18:181 - 7; http://dx.doi.org/10.1038/mt.2009.207; PMID: 19738602
- Wang F, Fu XD, Zhou Y, Zhang Y. Down-regulation of the cyclin E1 oncogene expression by microRNA-16-1 induces cell cycle arrest in human cancer cells. BMB Rep 2009; 42:725 - 30; http://dx.doi.org/10.5483/BMBRep.2009.42.11.725; PMID: 19944013
- Bueno MJ, Malumbres M. MicroRNAs and the cell cycle. Biochim Biophys Acta 2011; 1812:592 - 601; http://dx.doi.org/10.1016/j.bbadis.2011.02.002; PMID: 21315819
- Zhang X, Zhao X, Fiskus W, Lin J, Lwin T, Rao R, Zhang Y, Chan JC, Fu K, Marquez VE, et al. Coordinated silencing of MYC-mediated miR-29 by HDAC3 and EZH2 as a therapeutic target of histone modification in aggressive B-Cell lymphomas. Cancer Cell 2012; 22:506 - 23; http://dx.doi.org/10.1016/j.ccr.2012.09.003; PMID: 23079660
- Bui TV, Mendell JT. Myc: Maestro of MicroRNAs. Genes Cancer 2010; 1:568 - 75; http://dx.doi.org/10.1177/1947601910377491; PMID: 20882107
- Ivanovska I, Ball AS, Diaz RL, Magnus JF, Kibukawa M, Schelter JM, Kobayashi SV, Lim L, Burchard J, Jackson AL, et al. MicroRNAs in the miR-106b family regulate p21/CDKN1A and promote cell cycle progression. Mol Cell Biol 2008; 28:2167 - 74; http://dx.doi.org/10.1128/MCB.01977-07; PMID: 18212054
- Kim YK, Yu J, Han TS, Park SY, Namkoong B, Kim DH, Hur K, Yoo MW, Lee HJ, Yang HK, et al. Functional links between clustered microRNAs: suppression of cell-cycle inhibitors by microRNA clusters in gastric cancer. Nucleic Acids Res 2009; 37:1672 - 81; http://dx.doi.org/10.1093/nar/gkp002; PMID: 19153141
- Aqeilan RI, Calin GA, Croce CM. miR-15a and miR-16-1 in cancer: discovery, function and future perspectives. Cell Death Differ 2010; 17:215 - 20; http://dx.doi.org/10.1038/cdd.2009.69; PMID: 19498445
- Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M, Ferlito M, Lowenstein CJ, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell 2007; 26:745 - 52; http://dx.doi.org/10.1016/j.molcel.2007.05.010; PMID: 17540599
- Raver-Shapira N, Marciano E, Meiri E, Spector Y, Rosenfeld N, Moskovits N, Bentwich Z, Oren M. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell 2007; 26:731 - 43; http://dx.doi.org/10.1016/j.molcel.2007.05.017; PMID: 17540598
- Zhao X, Lwin T, Zhang X, Huang A, Wang J, Marquez VE, Chen-Kiang S, Dalton WS, Sotomayor E, Tao J. Disruption of the MYC-miRNA-EZH2 loop to suppress aggressive B-cell lymphoma survival and clonogenicity. Leukemia 2013; 27:2341 - 50; http://dx.doi.org/10.1038/leu.2013.94; PMID: 23538750
- Zhao JJ, Lin J, Lwin T, Yang H, Guo J, Kong W, Dessureault S, Moscinski LC, Rezania D, Dalton WS, et al. microRNA expression profile and identification of miR-29 as a prognostic marker and pathogenetic factor by targeting CDK6 in mantle cell lymphoma. Blood 2010; 115:2630 - 9; http://dx.doi.org/10.1182/blood-2009-09-243147; PMID: 20086245
- Knies-Bamforth UE, Fox SB, Poulsom R, Evan GI, Harris AL. c-Myc interacts with hypoxia to induce angiogenesis in vivo by a vascular endothelial growth factor-dependent mechanism. Cancer Res 2004; 64:6563 - 70; http://dx.doi.org/10.1158/0008-5472.CAN-03-3176; PMID: 15374969
- Sodir NM, Evan GI. Finding cancer’s weakest link. Oncotarget 2011; 2:1307 - 13; PMID: 22202195
- El Baroudi M, Corà D, Bosia C, Osella M, Caselle M. A curated database of miRNA mediated feed-forward loops involving MYC as master regulator. PLoS One 2011; 6:e14742; http://dx.doi.org/10.1371/journal.pone.0014742; PMID: 21390222
- Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E, Furth EE, Lee WM, Enders GH, Mendell JT, et al. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet 2006; 38:1060 - 5; http://dx.doi.org/10.1038/ng1855; PMID: 16878133
- Dang CV, Le A, Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res 2009; 15:6479 - 83; http://dx.doi.org/10.1158/1078-0432.CCR-09-0889; PMID: 19861459
- Liu W, Le A, Hancock C, Lane AN, Dang CV, Fan TW, Phang JM. Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c-MYC. Proc Natl Acad Sci U S A 2012; 109:8983 - 8; http://dx.doi.org/10.1073/pnas.1203244109; PMID: 22615405
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 2008; 7:11 - 20; http://dx.doi.org/10.1016/j.cmet.2007.10.002; PMID: 18177721
- Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA, Thompson CB. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell 2005; 8:311 - 21; http://dx.doi.org/10.1016/j.ccr.2005.09.008; PMID: 16226706
- Lin CH, Jackson AL, Guo J, Linsley PS, Eisenman RN. Myc-regulated microRNAs attenuate embryonic stem cell differentiation. EMBO J 2009; 28:3157 - 70; http://dx.doi.org/10.1038/emboj.2009.254; PMID: 19745813
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131:861 - 72; http://dx.doi.org/10.1016/j.cell.2007.11.019; PMID: 18035408
- Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 2007; 448:318 - 24; http://dx.doi.org/10.1038/nature05944; PMID: 17554336
- Liu C, Tang DG. MicroRNA regulation of cancer stem cells. Cancer Res 2011; 71:5950 - 4; http://dx.doi.org/10.1158/0008-5472.CAN-11-1035; PMID: 21917736
- Rao DS, O’Connell RM, Chaudhuri AA, Garcia-Flores Y, Geiger TL, Baltimore D. MicroRNA-34a perturbs B lymphocyte development by repressing the forkhead box transcription factor Foxp1. Immunity 2010; 33:48 - 59; http://dx.doi.org/10.1016/j.immuni.2010.06.013; PMID: 20598588
- Craig VJ, Cogliatti SB, Imig J, Renner C, Neuenschwander S, Rehrauer H, Schlapbach R, Dirnhofer S, Tzankov A, Müller A. Myc-mediated repression of microRNA-34a promotes high-grade transformation of B-cell lymphoma by dysregulation of FoxP1. Blood 2011; 117:6227 - 36; http://dx.doi.org/10.1182/blood-2010-10-312231; PMID: 21460242
- Peter ME. Let-7 and miR-200 microRNAs: guardians against pluripotency and cancer progression. Cell Cycle 2009; 8:843 - 52; http://dx.doi.org/10.4161/cc.8.6.7907; PMID: 19221491
- Sampson VB, Rong NH, Han J, Yang Q, Aris V, Soteropoulos P, Petrelli NJ, Dunn SP, Krueger LJ. MicroRNA let-7a down-regulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer Res 2007; 67:9762 - 70; http://dx.doi.org/10.1158/0008-5472.CAN-07-2462; PMID: 17942906
- Lin J, Lwin T, Zhao JJ, Tam W, Choi YS, Moscinski LC, Dalton WS, Sotomayor EM, Wright KL, Tao J. Follicular dendritic cell-induced microRNA-mediated upregulation of PRDM1 and downregulation of BCL-6 in non-Hodgkin’s B-cell lymphomas. Leukemia 2011; 25:145 - 52; http://dx.doi.org/10.1038/leu.2010.230; PMID: 20966935
- Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, Diehn M, Liu H, Panula SP, Chiao E, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell 2009; 138:592 - 603; http://dx.doi.org/10.1016/j.cell.2009.07.011; PMID: 19665978
- Bai JX, Yan B, Zhao ZN, Xiao X, Qin WW, Zhang R, Jia LT, Meng YL, Jin BQ, Fan DM, et al. Tamoxifen represses miR-200 microRNAs and promotes epithelial-to-mesenchymal transition by up-regulating c-Myc in endometrial carcinoma cell lines. Endocrinology 2013; 154:635 - 45; http://dx.doi.org/10.1210/en.2012-1607; PMID: 23295740
- Iliopoulos D, Lindahl-Allen M, Polytarchou C, Hirsch HA, Tsichlis PN, Struhl K. Loss of miR-200 inhibition of Suz12 leads to polycomb-mediated repression required for the formation and maintenance of cancer stem cells. Mol Cell 2010; 39:761 - 72; http://dx.doi.org/10.1016/j.molcel.2010.08.013; PMID: 20832727
- Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep 2008; 9:582 - 9; http://dx.doi.org/10.1038/embor.2008.74; PMID: 18483486
- Lin SL, Chang DC, Chang-Lin S, Lin CH, Wu DT, Chen DT, Ying SY. Mir-302 reprograms human skin cancer cells into a pluripotent ES-cell-like state. RNA 2008; 14:2115 - 24; http://dx.doi.org/10.1261/rna.1162708; PMID: 18755840
- Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S, et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol 2010; 12:247 - 56; PMID: 20173740
- Onnis A, De Falco G, Antonicelli G, Onorati M, Bellan C, Sherman O, Sayed S, Leoncini L. Alteration of microRNAs regulated by c-Myc in Burkitt lymphoma. PLoS One 2010; 5:5; http://dx.doi.org/10.1371/journal.pone.0012960; PMID: 20930934
- Zhang X, Chen X, Lin J, Lwin T, Wright G, Moscinski LC, Dalton WS, Seto E, Wright K, Sotomayor E, et al. Myc represses miR-15a/miR-16-1 expression through recruitment of HDAC3 in mantle cell and other non-Hodgkin B-cell lymphomas. Oncogene 2012; 31:3002 - 8; http://dx.doi.org/10.1038/onc.2011.470; PMID: 22002311
- Lwin T, Zhao X, Cheng F, Zhang X, Huang A, Shah B, Zhang Y, Moscinski LC, Choi YS, Kozikowski AP, et al. A microenvironment-mediated c-Myc/miR-548m/HDAC6 amplification loop in non-Hodgkin B cell lymphomas. J Clin Invest 2013; 123:4612 - 26; http://dx.doi.org/10.1172/JCI64210; PMID: 24216476
- Saito Y, Liang G, Egger G, Friedman JM, Chuang JC, Coetzee GA, Jones PA. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell 2006; 9:435 - 43; http://dx.doi.org/10.1016/j.ccr.2006.04.020; PMID: 16766263
- Paik JH, Jang JY, Jeon YK, Kim WY, Kim TM, Heo DS, Kim CW. MicroRNA-146a downregulates NFκB activity via targeting TRAF6 and functions as a tumor suppressor having strong prognostic implications in NK/T cell lymphoma. Clin Cancer Res 2011; 17:4761 - 71; http://dx.doi.org/10.1158/1078-0432.CCR-11-0494; PMID: 21610143
- Chang TC, Zeitels LR, Hwang HW, Chivukula RR, Wentzel EA, Dews M, Jung J, Gao P, Dang CV, Beer MA, et al. Lin-28B transactivation is necessary for Myc-mediated let-7 repression and proliferation. Proc Natl Acad Sci U S A 2009; 106:3384 - 9; http://dx.doi.org/10.1073/pnas.0808300106; PMID: 19211792
- Jiang X, Huang H, Li Z, Li Y, Wang X, Gurbuxani S, Chen P, He C, You D, Zhang S, et al. Blockade of miR-150 maturation by MLL-fusion/MYC/LIN-28 is required for MLL-associated leukemia. Cancer Cell 2012; 22:524 - 35; http://dx.doi.org/10.1016/j.ccr.2012.08.028; PMID: 23079661
- Mogilyansky E, Rigoutsos I. The miR-17/92 cluster: a comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ 2013; 20:1603 - 14; http://dx.doi.org/10.1038/cdd.2013.125; PMID: 24212931
- Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, Henderson JM, Kutok JL, Rajewsky K. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol 2008; 9:405 - 14; http://dx.doi.org/10.1038/ni1575; PMID: 18327259
- Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 2008; 132:875 - 86; http://dx.doi.org/10.1016/j.cell.2008.02.019; PMID: 18329372
- He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, et al. A microRNA polycistron as a potential human oncogene. Nature 2005; 435:828 - 33; http://dx.doi.org/10.1038/nature03552; PMID: 15944707
- Cannell IG, Bushell M. Regulation of Myc by miR-34c: A mechanism to prevent genomic instability?. Cell Cycle 2010; 9:2726 - 30; http://dx.doi.org/10.4161/cc.9.14.12182; PMID: 20603603
