Abstract
In Drosophila melanogaster (D. melanogaster), neurosecretory insulin-like peptide-producing cells (IPCs), analogous to mammalian pancreatic b cells are involved in glucose homeostasis. Extending those findings, we have developed in the adult fly an oral glucose tolerance test and demonstrated that IPCs indeed are responsible for executing an acute glucose clearance response. To further develop D. melanogaster as a relevant system for studying age-associated metabolic disorders, we set out to determine the impact of adult-specific partial ablation of IPCs (IPC knockdown) on insulin-like peptide (ILP) action, metabolic outcomes and longevity. Interestingly, while IPC knockdown flies are hyperglycemic and glucose intolerant, these flies remain insulin sensitive as measured by peripheral glucose disposal upon insulin injection and serine phosphorylation of a key insulin-signaling molecule, Akt. Significant increases in stored glycogen and triglyceride levels as well as an elevated level of circulating lipid measured in adult IPC knockdown flies suggest profound modulation in energy metabolism. Additional physiological outcomes measured in those flies include increased resistance to starvation and impaired female fecundity. Finally, increased life span and decreased mortality rates measured in IPC knockdown flies demonstrate that it is possible to modulate ILP action in adult flies to achieve life span extension without insulin resistance. Taken together, we have established and validated an invertebrate genetic system to further investigate insulin action, metabolic homeostasis and regulation of aging regulated by adult IPCs.
Introduction
Metabolism in multicellular organisms influences many aspects of physiological regulation, including growth and development, reproductive fitness, energy homeostasis and aging.Citation1 With the goal of better understanding the interactions between metabolism and aging in animals, significant progress has been made in using invertebrate genetic model systems including C. elegans and D. melanogaster with an emphasis on the conserved insulin/IGF signaling (IIS) pathway.Citation2–Citation7
Glucose homeostasis is maintained in a remarkably conserved manner in D. melanogaster, fruit flies. Functionally analogous to the insulin-secreting pancreatic β cells and glucagon-secreting pancreatic islet α cells that act in opposition to maintain glucose homeostasis in mammals, endocrine tissues insulin-like peptide-producing cells (IPCs) and adipokinetic hormone (AKH)-producing corpora cardiaca (CC) cells in the fly have been shown to function in glucose sensing.Citation8,Citation9 Genetic manipulation leading to IPC ablation in the brain mimics a diabetic phenotype, with increased sugar levels in larval and adult hemolymph associated with growth retardation, developmental delay and reduced fecundity.Citation8,Citation10 Conversely, targeted ablation of AKH-producing CC cells renders larvae and adults hypoglycemic.Citation9,Citation11 Seven Drosophila insulin-like peptides (DILPs) have been identified with five of them (DILPs 1–5) highly homologous to their mammalian counterparts.Citation12 Of these five DILPs, DILP2, 3 and 5 are produced in IPCs and are involved in growth, metabolism and longevity regulation.Citation8,Citation10,Citation13 While these studies have demonstrated the importance of DILPs in maintaining glucose homeostasis, the precise regulatory roles of DILPs during adulthood must be delineated in order to better study age-associated metabolic diseases, such as type 2 diabetes in the fly.
To fully develop fruit flies as such a model system, it is essential to understand the molecular mechanism responsible for DILP release in IPCs as well as systemic DILP action. We have recently demonstrated that the mechanism for DILP secretion, like that found in pancreatic β cells, may also involve KATP channels.Citation14 Specifically, exposure to glucose or the sulfonylurea KATP channel blocker glibenclamide depolarizes membrane potential of adult IPCs. As opening of voltage-gated Ca2+ channels and influx of Ca2+ ultimately leads to insulin secretion in β cells, a robust increase in intracellular Ca2+ levels in IPCs in response to glucose or glibenclamide was also detected.Citation14 While these electrophysiological studies have suggested a likely conserved mechanism of DILP secretion by adult IPCs, additional functional measurements to determine peripheral insulin responses including glucose tolerance and insulin sensitivity need to be established in order to fully develop fruit flies as a model system to assess insulin action.
Here we report the development of an oral glucose tolerance test (OGTT) in adult flies where a remarkably similar glucose clearance profile to that seen in mammals is measured. Furthermore, we demonstrate the effect of a high calorie diet, intact AKH producing CCs or intact IPCs has on glucose clearance response in these animals. Building on these physiological assays, and to address the aging factor regarding DILP action, we employed an IPC-specific, conditional driver to partially ablate IPCs only during adulthood as confirmed by real-time expression analysis on three transcripts known to be produced in IPCs (dilp2, dilp3 and dilp5).Citation10 Therefore, these flies will be termed “adult IPC knockdown (KD)” flies. We show that while adult IPC KD flies possess a diabetic phenotype, they remain insulin sensitive as demonstrated by an insulin tolerance test and serine phosphorylation of Akt in the peripheral flight muscle tissue. A major role of adult IPCs in energy metabolism is validated by altered glycogen and triglyceride stores as well as increased circulating lipid levels measured in adult IPC KD flies. Finally, we show that physiological outcomes including female fecundity, stress response and longevity are also significantly modulated in adult IPC KD flies. Taken together, we have developed a model system that reveals complex outcomes in energy homeostasis, stress response, female fecundity and longevity regulation as the consequence of adult-specific attenuation of dilps.
Results
Adult IPCs confer glucose tolerance response in the fly.
To fully develop a fly model for metabolic disorders affecting glucose homeostasis, it is important to measure the organism's ability to clear peripheral sugar load following ingestion of carbohydrates. Similar to an oral glucose tolerance test (OGTT) administered in humans,Citation15 we subjected replicate groups of adult flies to fasting and subsequent ingestion of glucose or sucrose solutions and measured their glucose disposal rates. As shown in , ingestion of glucose results in typical glucose clearance kinetics as measured in rodent models following intraperitoneal glucose injection.Citation16 Ingestion of sucrose, the carbohydrate source in the diet under laboratory conditions, results in comparable glucose clearance profiles (Suppl. Fig. 1A). These results demonstrate a measurable physiological outcome reflecting glucose homeostasis at a whole animal level. Having established a functional OGTT in flies reared on standard diet, we then asked whether alterations in calorie intake would impact on glucose tolerance response. To address this question, control flies were maintained on low calorie (5% yeast, 5% sucrose) or high calorie (20% yeast, 20% sucrose) diet for twenty days and subjected to OGTT. As shown in , flies reared on high calorie diet have increased fasting hemolymph glucose levels and slower glucose clearance kinetics as compared to flies raised on a low calorie diet. These results present another similarity in metabolic response between fly and mammal where a high calorie diet and resulting obesity are risk factors for glucose intolerance.Citation17
While it has been shown in larvae that ablation of IPCs and CCs results in hyperglycemia and hypoglycemia, respectively, functional contribution of those neurosecretory cells to glucose tolerance response in the adult fly has not been determined.Citation8,Citation9 To measure glucose tolerance response as the result of constitutive ablation of CCs, the AKH-Gal4 driver was used to drive the expression of the pro-apoptotic gene, reaper in CCsCitation9 whereas the dilp3-Gal4 driver was employed to drive the reaper expression in IPCsCitation14 for subsequent glucose tolerance response measurements. Interestingly, while flies with genetic ablation of CCs are capable of clearing peripheral glucose load to the same extent as measured in control flies (), ablation of IPCs significantly impairs the ability of those flies to clear the glucose load and renders those flies glucose intolerant (). These results are consistent with the functional similarity between AKH-secreting CCs and glucagon-secreting islet α cells whereas IPCs maintain glucose homeostasis by secreting DILPs to control peripheral glucose clearance. Thus, by creating partial, constitutive ablation of IPCs as confirmed by reduced expression levels of IPC-specific dilps,Citation14 our studies have provided physiological evidence for a role of IPCs in controlling glucose homeostasis. However, such approaches may not discern whether lack of full IPC function during development may contribute to glucose intolerance measured in adults. To restrict IPC dysfunction to the largely post-mitotic adulthood, we acquired a conditional, IPC-specific dilp2-GeneSwitch driver (a kind gift from Dr. H Jasper, University of Rochester). We first validated the functionality of this conditional driver by demonstrating IPC-specific GFP expression in the dilp2-GeneSwitch/UAS-GFP adult flies only when 200 mM RU-486 was added to the diet (Suppl. Fig. 2A).Citation18 To create adult-specific IPC ablation, we again used the UAS-reaper line to generate dilp2-GeneSwitch/UAS-reaper flies. In the presence of RU-486 containing diet, real-time expression analysis was employed to quantify IPC-specific dilp2, dilp3 and dilp5 transcript levels as a read-out for the extent of IPC ablation in those flies.Citation14 As shown in Supplementary Figure 2B, an average of 50% decrease in IPC-specific dilp2, dilp3 and dilp5 expression was achieved suggesting partial destruction of adult IPCs. To ask the question of whether or not adult IPC KD flies were affected in their response to OGTT, we measured glucose clearance responses of dilp2-GeneSwitch/UAS-reaper along with control dilp2-GeneSwitch/w1118 flies raised on RU-486 or diluent containing diet since day 1 of their adulthood for 14 days prior to OGTT. As shown in , fasting hyperglycemia and a much slower glucose clearance response is measured in dilp2-GeneSwitch/UAS-reaper flies fed with RU-486 containing diet as compared to genetically identical flies raised on diluent containing diet. We ruled out any potential non-specific, RU-486 effect on glucose tolerance response as similar glucose clearance kinetics were observed between control dilp2-GeneSwitch/w1118 flies reared on RU-486 or diluent containing diet (Suppl. Fig. 1B). Taken together, we have demonstrated that adult-specific partial IPC ablation is sufficient to negatively affect glucose homeostasis at the whole animal level as reflected by both fasting hyperglycemia and impaired glucose tolerance response. To further develop our genetic model and understand the physiological impact of attenuated production of adult DILPs, additional studies reported henceforth were focused on the characterization of adult-specific IPC knockdown (IPC KD) flies.
Adult-specific IPC KD flies are insulin sensitive.
We next investigated whether fasting hyperglycemia and glucose intolerance measured in adult IPC KD flies is associated with insulin resistance as is often seen in type 2 diabetes.Citation19 To approach this question, we developed an insulin tolerance assay in the adult fly based on mammalian proceduresCitation20 where adult flies were injected with bovine insulinCitation21 followed by hemolymph extraction and glucose measurements. As shown in , a 29% decease in circulating glucose levels was measured as the result of insulin injection in control flies raised on RU-486 containing diet for 14 days whereas a comparable, 22% decease in circulating glucose levels was measured as the result of insulin injection in adult IPC KD flies (under RU-486 induction). In contrast, when injected with PBS only, replicate flies reared under the same condition showed no change in circulating glucose concentration 30 minutes post-injection (). Thus, while adult-specific, partial IPC ablation is sufficient to disrupt glucose homeostasis, those flies remain responsive to insulin injection in the disposal of circulating glucose. To further understand the state of insulin sensitivity in adult IPC KD flies on the cellular level, we sought to determine the phosphorylation status of the serine/threonine kinase Akt involved in the insulin signaling pathway. In the mammalian system, peripheral insulin resistance is determined by the number of available insulin receptors, their substrate (IRS) or the phosphoylation status of the receptor or downstream components of the insulin signaling pathway.Citation16 Akt is a key kinase responsible for retaining the transcriptional factor FoxO in the cytoplasm following activation of the insulin receptor.Citation22 To assess insulin signaling in the periphery in our model system, the phosphorylation status of Akt isolated from thoracic flight muscles was examined. As shown in , a comparable level of steady-state serine phosphorylation was detected in adult IPC KD flies under RU-486 induction as compared to genetically identical flies reared on diluent containing diet (-RU) or control flies raised under the same conditions. Furthermore, using a similar molecular approach, we show that while background levels of phosphorylated Akt were detected in flies under fasting conditions, i.e., much reduced insulin signaling; re-feeding of glucose resulted in an increase in Akt phosphorylation, i.e., full insulin signaling. Increase Akt phosphrylation in response to re-feeding was evident in both control and adult IPC KD flies under RU-486 induction (Suppl. Fig. 3). These results provide additional support for both the validity of our approach in using Akt phosphorylation as a read-out for insulin signaling in our model system and an independent line of evidence for insulin sensitivity in adult IPC KD flies.
Adult-specific partial IPC ablation affects glycogen and lipid metabolism.
Our findings of hyperglycemia and glucose intolerance but not insulin resistance measured in adult IPC KD flies strongly suggest modulated DILP action as the result of partial adult IPC ablation. To understand how the modulation of DILP action influences energy metabolism, we determined if homeostasis of two major energy stores, glycogen and triglyceride was affected. Fourteen-day-old adult flies raised on RU-486 or diluent containing diet since eclosion were used for body composition measurements. As shown in , a dramatic increase of 86% in glycogen stores is detected as the result of partial adult IPC ablation whereas cellular glucose and trehalose storage remains unaltered (data not shown). To understand the impact on lipid metabolism in those flies, we measured both circulating and stored triglyceride content. As shown in , a moderate but statistically significant increase of 23% in cellular lipid storage is measured in adult IPC KD flies under RU-486 induction as compared to genetically matched control flies under the same condition or a cohort of dilp2-GeneSwitch/UAS-reaper flies fed with diluent containing diet. Consistent with the notion that lipid metabolism is affected in adult IPC KD flies, a 50% increase in circulating triglyceride is measured in adult IPC KD flies ().
Adult-specific ablation of IPCs extends life span, modulates stress response and regulates female fecundity.
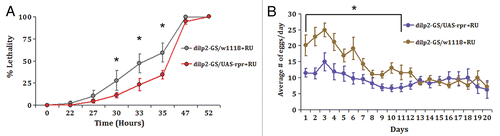
To investigate how adult-specific partial IPC ablation may impact life span control, we performed survivorship studies on adult IPC KD along with genetically matched control flies reared on diluent or RU-486 containing food since day 1 of adulthood. While a full statistical analysis of the two life span trials is shown in Supplementary Table 1, representative survivorships and mortality curves from one trial are shown in . In this life span trial, we measured an increase of 24% in both female mean and maximum life spans in adult IPC KD flies as compared to genetically identical flies raised on diluent containing diet (). In male IPC KD flies, a moderate increase of 15 and 7% in mean and maximum life span, respectively was measured in adult IPC KD flies as compared to genetically identical flies raised on diluent containing diet (). As observed in our previous studies, the consumption of RU-486 containing diet also had a small effect (∼±7% change in life span) on control flies without the UAS construct (Suppl. Table 1).Citation18 When taking this effect into consideration, a moderate, overall life span extension remains with 14% and 16% calculated in female and male adult IPC KD flies, respectively. Importantly, analysis of mortality curves reveals a slower rate of aging of adult IPC KD flies as compared to genetically matched flies under RU-486 induction ( and D). Similar analysis was performed on adult IPC KD flies and genetically identical flies without RU-486 induction demonstrating a moderate decrease in mortality rates (Suppl. Fig. 4). These results lend further significance and relevance to our model and approaches in understanding adult-specific IPC manipulation and its impact on the regulation of aging.
Reduced insulin signaling by mutations of the components of the IIS pathway often leads to increased stress resistance.Citation10 Interestingly, while adult IPC KD flies are resistant to starvation (), no increased resistance to oxidative damage compared to controls was observed in those flies (data not shown). Finally, insulin signaling in the mammalian central nervous system is known to regulate reproduction.Citation23 Constitutive partial ablation of Drosophila IPCs results in a 2-fold reduction in egg production.Citation10 To assess the reproductive activity in female IPC KD flies, we found a pronounced, up to a 2-fold reduction in average egg production only in the first 10 days of their reproductive life as the result of adult-specific partial IPC ablation ().
Discussion
While D. melanogaster has proven to be a powerful genetic model system in understanding the importance of the IIS pathway in metabolism and aging,Citation2–Citation5 its potential utility in elucidating molecular mechanisms in regulating insulin action has not been fully explored. To establish fruit flies as a relevant model for metabolic disorders, we have developed physiological assays to monitor glucose homeostasis and insulin sensitivity in the adult fly. Mirroring the OGTT administered in humans, adult fruit flies exhibit remarkably similar peripheral glucose clearance kinetics following the ingestion of a bolus of sugar solution. We show that this glucose tolerance response is dependent upon the full complement of the IPCs as partial, constitutive ablation of those cells renders flies hyperglycemic and glucose intolerant. On the other hand, loss of AKH-secreting CCs has little effect on maintaining circulating glucose homeostasis. This is consistent with previous reports that AKH contributes to hemolymph sugar homeostasis mainly by mobilizing sugar and lipids from the fat body during energy-requiring activities, such as flight or locomotion.Citation24–Citation26 We have recently demonstrated that exposure to glucose depolarizes membrane potential of adult IPCs and this effect is mimicked with the sulfonylurea glibenclamide, a pharmacological blocker known to inhibit KATP channels for insulin release in pancreatic β cells.Citation14 Thus, a conserved insulin secreting mechanism in Drosophila IPCs is likely responsible for the acute uptake of circulating glucose in maintaining homeostasis.
To further develop fruit flies to model age-associated metabolic disorders, here we demonstrate that adult-specific, partial ablation of IPCs is sufficient to cause fasting hyperglycemia and glucose intolerance. As indicated by the quantitative RT-PCR analysis, an approximately 50% reduction of IPC-produced dilps is detected in our adult IPC KD flies. Reduced circulating insulin levels are frequently associated with insulin resistance.Citation16 However, calorie restriction leading to decreased insulin levels is thought to improve insulin sensitivity, delay age-associated pathologies and extend life span.Citation27,Citation28 We found that in adult IPC KD flies insulin sensitivity is largely intact as assessed by both unaltered Akt phosphorylation detected in peripheral flight muscle tissue and the ability of those flies to dispose of circulating glucose in response to insulin injection. Two conclusions may be drawn from those results. First, while adult IPC KD flies suffer from fasting hyperglycemia and glucose intolerance likely due to an insufficient central supply of DILPs, their peripheral insulin signaling capacities leading to glucose uptake remain intact in response to exogenous insulin injection. Second, although reduced levels of endogenous DILPs are adequate for activating the IIS biochemical machinery as evidenced by Akt phosphorylation, the ability of the adult IPC KD flies to maintain glucose homeostasis is compromised. Interestingly, similar outcomes of altered glucose homeostasis have been reported as the result of deficiency of the insulin receptor in the brown adipose tissue.Citation16 The resulting BATIRKO knock-out mice exhibited reduced plasma insulin levels, fasting hyperglycemia, age-associated glucose intolerance and peripheral insulin sensitivity.Citation16 Our findings thus reveal potentially complex control mechanisms in governing DILP action to maintain glucose homeostasis in the adult fly.
Constitutive, partial ablation of IPCs has been shown to affect energy metabolism.Citation10 Similarly, we demonstrate that adult-specific, partial IPC ablation significantly alters lipid metabolism and glycogen storage. In addition to increased triglyceride stores, we also detected increased circulating lipid levels mimicking increased plasma triglyceride and free fatty acids associated with β-cell dysfunction.Citation29–Citation31 Our results underscore the profound importance of adult IPCs in controlling energy metabolism and lend further support in developing a relevant invertebrate model system for studying metabolic disorders. Increased lipid content has been associated with heightened starvation resistance and increased life span as exemplified by genetic mutations known to affect the IIS and TOR pathways.Citation32–Citation34 Consistently, adult-specific IPC KD flies are also more starvation resistant than controls and exhibit a moderate life span extension in both females and males. While our results are in accordance with current notions on the correlation between lipid content and longevity determination, the exact underlying mechanism for altered lipid metabolism and increased life span measured in adult IPC KD flies remains to be elucidated.
Interestingly, while constitutive, partial ablation of IPCs results in increased resistance to oxidative stress, we observed no such response in adult IPC KD flies when exposed to paraquat, a free radical generator. One possibility is that the more severe attenuation in the expression of dilps achieved as the result of constitutive IPC ablation (∼70% reduction vs. 50% reduction seen in adult IPC KD flies) may engender a physiological outcome allowing increased oxidative stress resistance. Alternatively, specific or more dramatic physiological changes resulting from early-life IPC knockdown may be required for the enhanced adult resistance to certain forms of stress as seen with constitutive IPC ablation. Another differential physiological response between constitutive, partial IPC ablation and adult IPC knockdown is reflected in female fecundity. Similar to the reduced female fecundity resulting from constitutive IPC ablation, a significant reduction in egg production is also observed in adult IPC KD flies. However, unlike the persistent impairment in fecundity seen as the result of constitutively ablated flies,Citation10 comparable levels in egg production between adult IPC KD and control females were restored after the first 10 days of their reproductive life. This is due to the fact that while control females experience age-associated decline in egg production, the adult IPC KD females appear to maintain a constant level of egg production during the first 20 days of their adult life. Thus, adult DILPs may be crucial in facilitating the initial burst of egg production seen in control flies. The reproductive period in Drosophila females exhibits three distinct epochs based on mating-dependent egg production and mortality.Citation35 Therefore, it is likely that normal titers of DILPs may be required for the highest levels of egg production that normally occur during the early optimal epoch in female flies.
Our findings that adult-specific deficiency of dilps is sufficient to moderately extend life span argue for the possibility of modulating DILP action to achieve life span extension without insulin resistance. Large longevity extension has been achieved in both C. elegans and D. melanogaster through loss-of-function mutations of the IIS pathway molecules.Citation2,Citation32,Citation33,Citation36,Citation37 However, a concern remains that such extension may be achieved at the expense of insulin resistance that has not been adequately addressed in those model systems. In mammals, loss-of-function mutations in the insulin receptor lead to insulin resistance and shortened life span.Citation38 On the other hand, selective loss of the intact insulin receptor in the mouse adipose tissue (FIRKO mice) results in extended life span without diabetic consequences.Citation39 Because of the reduced adiposity and enhanced glucose tolerance response measured in FIRKO mice, it was postulated that selective loss of insulin signaling in the adipose tissue may mimic calorie restriction conditions,Citation39 conditions shown to decrease circulating insulin.Citation27,Citation28 In our model system, we demonstrate that reduced DILP production only during adulthood engenders differential regulation in aspects of glucose homeostasis mimicking the complex process seen in other, more complex animal models substantiating the use of an invertebrate model for studying relevant insulin action affecting systemic energy metabolism and longevity. With a set of molecular tools for monitoring systemic glucose homeostasis and insulin action in the adult fly established and validated, the identification and characterization of potentially novel molecular interventions that seek to regulate glucose homeostasis, energy metabolism and longevity should be permitted.
Materials and Methods
Generation and maintenance of fly stocks.
To partially ablate IPCs in a constitutive manner, the dilp3-Gal4 driver was used to drive the expression of the pro-apoptotic gene, reaper in IPCs.Citation18 The AKH-Gal4 driver was used to drive reaper expression to achieve constitutive ablation of CCs. To obtain adult-specific IPC ablation, the conditional dilp2-GeneSwitch driver was used to drive reaper expression in adult IPCs in an RU-486-dependent manner.Citation18 The dilp3-Gal4 driver line obtained from Dr. M. Tatar (Brown University) has previously been characterized.Citation18 The AKH-Gal4 driver line was obtained from Dr. S. Kim (Stanford University). The dilp2-GeneSwitch driver line was obtained from Dr. H. Jasper (University of Rochester). The UAS-reaper line (5824) was obtained from the Bloomington Stock Center. All fly stocks were maintained in a humidified, temperature-controlled incubator with 12 h on/off light cycle at 25°C on standard corn meal/yeast/sucrose/agar diet.
Oral glucose tolerance test, hemolymph collection and glucose measurements.
Adult flies were fasted for 16 hours on 1% agar before transferred to vials containing 10% glucose solution-soaked filters for 1 hour. While this group of flies (20–30) was subjected to hemolymph extraction,Citation18 replicate groups having consumed 10% glucose solution for 1 hour were then transferred to vials containing water-soaked filters for 30′ and 60′, respectively prior to hemolymph extraction. Multiple (4–7) experiments were performed with 20–30 flies/hemolymph extraction in each experiment. The amount of circulating glucose was measured using the Infinity Glucose Reagent (Thermo Electron Corporation).Citation14
Bovine insulin injection and insulin tolerance assay.
Injections were performed using a manual microinjector (Sutter Instruemt, Novato, CA) and 1.0 mm standard glass needles pulled to a fine tip with a model P-87 Flamming/Brown micropipette puller (Sutter Instruments, Novato, CA). Flies were injected through the left prescutum into flight muscle tissue after CO2 anesthesia and brief immobilization on ice. Experimental groups were injected with 0.1 µl of 0.01 mg/ml bovine insulin (Sigma) in PBS whereas control groups were injected with 0.1 µl of PBS alone.Citation21 Injected flies were allowed to recover in vials containing 1% agar for 5′ or 30′ before subjected to hemolymph extraction and glucose measurements. For each experiment, triplicates of 4 flies were injected and measured for hemolymph glucose levels. Three independent experiments were performed to obtain a total of 9 independent measurements.
Western blot analysis.
The steady-state Akt phosphorylation was determined in the protein lysates prepared from isolated thoracic flight muscle tissues. Control and IPC KD flies were raised on standard diet containing 200 µM RU-486 for 14 days prior to isolation of thoraces. Equal amounts of protein (100 µg) extracted using RIPA buffer in the presence of phosphatase inhibitors (Cell Signaling Technology) were analyzed by SDS-PAGE followed by western blot analysis using an Akt antibody (#9272, Cell Signaling Technology) and a phospho-Drosophila Akt (Ser505) antibody (#4054, Cell Signaling Technology) according to manufacturer's protocols.
Quantitative real-time RT-PCR expression analysis.
Total RNA was isolated from heads of 14-day old females raised on diluent or RU-486 containing food using the TRIzol method (Invitrogen). Subsequent cDNA and real-time RT-PCR experiments were performed as described previously.Citation14 Three independent RNA preparations with triplicates in each experiment were used to derive the mean ratios of target gene expression against the reference gene GAPDH. The primers for detecting the expression of dilp2, dilp3, dilp5 and GAPDH are described previously.Citation14
Glycogen and triglyceride body composition determination.
Whole body homogenates from 14-day-old male and female flies reared on RU-486 or diluent containing diet since eclosion were prepared as described.Citation14 For each assay, triplicates of 10 µg of homogenate for each sample were included. Glycogen content was calculated by subtracting the total glucose composition without amyloglucosidase (Sigma) digestion from the total glucose composition after amyloglucosidase digestion.Citation14 To measure triglyceride stores, fly homogenates (10 µg) were similarly prepared and subjected to analysis using the Infinity Triglycerides Reagent (Thermo Electron Corporation). To measure fasting circulating triglyceride, flies were fasted for 16 hours on 1% agar prior to hemolymph extraction. The triglyceride levels were determined using the Infinity Triglycerides Reagent. For each experiment, 20–30 flies were subjected to each hemolymph extraction and multiple (3–6) experiments were performed.
Life span and stress resistance studies.
To perform life span studies, homozygous virgins bearing the UAS-reaper transgene and control w1118 female virgins were crossed to dilp2-GeneSwitch driver males. The progeny from these crosses were maintained on standard corn meal/yeast/sucrose/agar diet containing 200 uM RU486 or diluent (ethanol) and passed to fresh vials every other day.Citation18 Two independent trials were performed. Numbers of flies included in each trial are indicated in Supplementary Table 1.
Both starvation and paraquat resistance assays were conducted as described previously.Citation18 Briefly, 14-day-old dilp2-GeneSwitch/UAS-reaper and dilp2-GeneSwitch/w1118 flies raised on RU-486 containing diet were placed in vials containing 1% agar (starvation assay) or a solution of 20 mM paraquat in 5% sucrose (paraquat assay) and the number of dead flies counted every 4–14 hours. Three independent experiments were performed. Each experiment used five to eight vials with 20 males or 20 females in each vial (100–160 males and 100–160 females per experiment).
Female fecundity.
Female fecundity was determined from daily counts of eggs produced by 20 individual females in single mating pairs of dilp2-GeneSwitch/UAS-reaper and dilp2-GeneSwitch/w1118 flies raised on RU-486 containing diet. The flies were passed to new vials every day and the number of eggs laid was counted and recorded for the first 20 days of their adult life.Citation14
Statistical analysis.
Statistical analysis for independent life span trials was performed using log-rank test (StatView). Age-specific mortality is calculated as 2ln(px) where px is the probability of surviving to age x. Mortality rates are presented on the natural log scale. Results for all other assays were analyzed using paired Student's t test.
Figures and Tables
Figure 1 Fasting glucose levels and oral glucose tolerance in adult flies. Except for flies (20-day-old) used in (B), 14-day-old adult flies of specified genotype were fasted for 16 hours on 1% agar before subjected to OGTT (see Materials and Methods). (A) Control w1118 flies show similar glucose clearance kinetics as seen in mammals. (B) High calorie diet results in fasting hyperglycemia and impaired glucose clearance. Adult w1118 flies were reared on high calorie (2.0 N; 20% sucrose and 20% yeast) or calorie restricted (0.5 N; 5% sucrose and 5% yeast) diet for twenty days prior to OGTT. (C) Constitutive ablation of AKH-secreting CCs (AKH-Gal4/UAS-reaper) has no negative effect on glucose tolerance response as compared to control AKH-Gal4/w1118 flies. (D) Constitutive ablation of IPCs renders those flies (dilp3-Gal4/UAS-reaper) hyperglycemic and glucose intolerant when compared to control dilp3-Gal4/w1118 flies. (E) Adult-specific, partial ablation of IPCs renders those flies (dilp2-GeneSwitch/UAS-reaper) hyperglycemic and glucose intolerant as compared to control dilp2-GeneSwitch/w1118 flies. dilp2-GeneSwitch/UAS-reaper (dilp2-GS/UAS-rpr) and control dilp2-GS/w1118 flies were reared on 200 µM RU-486 (RU) containing diet for 14 days prior to OGTT. Each value represents mean ± S.E.M. (N = 4–7 independent hemolymph collections with 20–30 flies/collection). *p < 0.05, **p < 0.02 (Student's t-test).
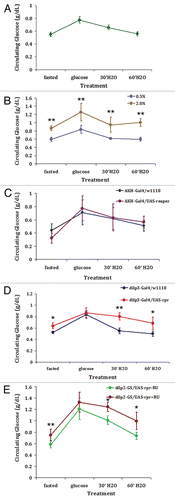
Figure 2 Adult-specific IPC KD flies remain insulin sensitive. (A) Similar glucose disposal responses are measured in control and adult IPC KD flies in an insulin tolerance test. Control (dilp2-GS/w1118) or IPC KD (dilp2-GS/UAS-rpr) flies reared on RU-486 containing diet for 14 days were injected with bovine insulin (1 ng in PBS) or PBS only. Flies were then allowed to recover for 5 or 30 minutes and circulating glucose levels were measured (see Materials and Methods). Circulating glucose measured in flies 5′ post-injection was arbitrarily set at 100% and the average % decrease in circulating glucose 30′ post-injection with pBS or insulin (Ins) is shown. A 5′ post-injection period in flies recovering from chill immobilization allows for adequate mixing of injected solution with hemolymph while minimizing potential insulin action on peripheral cells and tissues. A 29% decrease in circulating glucose following insulin injection was measured in control flies and a 22% decrease measured in adult IPC KD flies. Each bar represents mean ± S.E.M. (N = 9 independent hemolymph collections with 4 flies/collection). *p < 0.05 (Student's t test). (B) Steady-state serine phosphorylation of Akt is detected in adult IPC KD flies. Equal amounts (100 µg) of protein extracts isolated from thoracic flight muscles of control dilp2-GS/w1118 or dilp2-GS/UAS-rpr flies reared on RU-486 (+RU) or diluent (ethanol; −RU) containing diet for 14 days were separated by SDS-PAGE and analyzed by western blot with an anti-phospho-Drosophila Akt (Ser505) antibody (#4054, Cell Signaling Technology). The 65 kDa phsphoryated Akt is shown. Three biological repeats were carried out with a representative experiment shown.
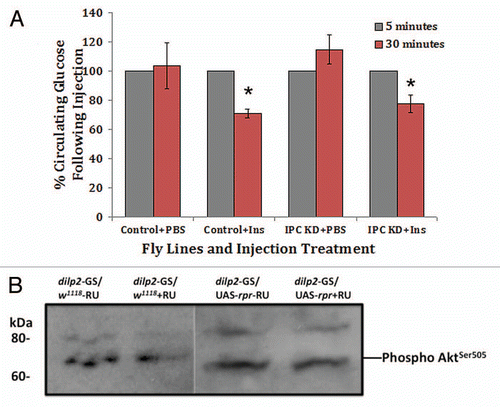
Figure 3 Partial ablation of adult IPCs modulates energy metabolism. Body composition analysis of adult IPC KD (dilp2-GS/UAS-rpr) and control (dilp2-GS/w1118) flies reared on 200µM RU-486 (+RU) or diluent (ethanol; −RU) containing diet for 14 days. (A) An average of 86% increase in glycogen storage is the result of partial ablation of adult IPCs. (B) An average of 23% increase in stored triglyceride is measured in adult IPC KD flies as compared to controls. The difference in triglyceride content between dilp2-GS/w1118−RU and dilp2-GS/w1118+RU flies is not statistically significant (p = 0.067). (C) An average of 50% increase in circulating triglyceride is measured in adult IPC KD flies as compared to controls. Each bar represents mean ± S.E.M. N = 3–5. *p = 0.02; **p < 0.01 (Student's t-test).
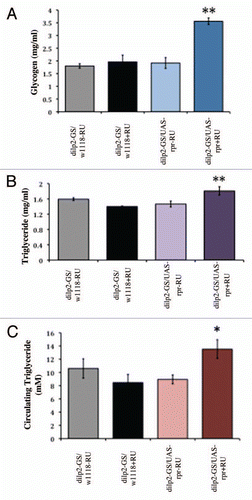
Figure 4 Partial ablation of adult IPCs extends life span by decreasing mortality rates. Survivorship curves for female (A) and male (B) flies are shown. Two independent trials were performed and results from one trial are shown. (A) Female mean life spans are 45 for control flies (dilp2-GS/w1118) and 45 for adult IPC KD flies (dilp2-GS/UAS-reaper) reared on diluent (ethanol; −RU) containing diet since eclosion whereas mean life spans are 48 for control flies and 56 for adult IPC KD flies reared on 200 µM RU-486 containing diet (+RU). (B) Male mean life spans are 41 for controls and 40 for adult IPC KD flies reared on diluent (ethanol; −RU) containing diet since eclosion whereas mean life spans are 39 for control flies and 46 for adult IPC KD flies reared on RU-486 containing diet (+RU). Log rank analysis shows a 17% increase in mean life span in female with partial adult IPC ablation as compared to controls under the same RU-486 treatment and an average of 18% increase in male (Suppl. Table 1). Age-specific mortality analysis of female (C) and male (D) from the same life span trial is shown as a comparison between dilp2-GS/UAS-reaper and dilp2-GS/w1118 flies raised on RU-486 containing diet. Natural log of the mortality rate (lx) is plotted. Administration of RU-486 or diluent (ethanol) was begun on the day of eclosion and continued throughout adult life as described.Citation18
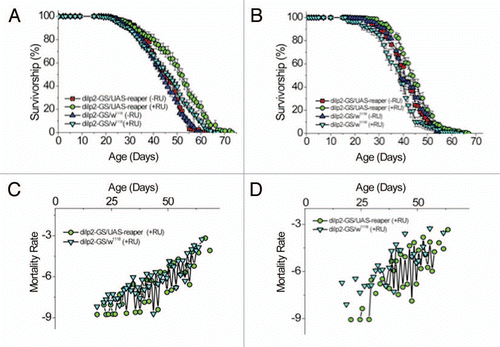
Figure 5 Partial ablation of adult IPCs increases starvation resistance and impairs female fecundity. (A) Adult IPC KD flies are more resistant to starvation than controls. Fourteen-day-old control (dilp2-GS/w1118) and adult IPC KD (dilp2-GS/UAS-reaper) flies raised on RU-486 containing diet since eclosion were placed in 1% agar vials and the number of dead flies was counted at noted time intervals. No difference in survival was observed between control and IPC KD flies raised on diluent (ethanol) containing diet (data not shown). Three independent assays were performed and a representative experiment is shown for males. Similar differences were seen for males and females. All values are presented as mean ± S.E.M. *p < 0.05. Each experiment included 5–8 vials with 20 flies in each vial, total of 100–160 flies for each condition. (B) Impaired female fecundity in the early reproductive period is the result of adult-specific partial IPC ablation. Virgin control (dilp2-GS/w1118) and adult IPC KD (dilp2-GS/UAS-reaper) females were placed in vials with RU-486 containing diet as single mating pairs. Average number of eggs per day for 20 individual females was determined from daily counts of eggs produced.Citation18 Two independent experiments were performed with similar results. Results from one representative experiment are shown. All values are presented as mean ± S.E.M. *p < 0.03. No difference in egg production was observed between control and adult IPC KD flies raised on diluent (ethanol) containing diet (data not shown).
Additional material
Download Zip (384.3 KB)Acknowledgements
We thank Dr. H. Jasper (University of Rochester) for the kind gift of dilp2-GeneSwitch driver flies. We also thank Dr. Adolfo Sánchez-Blanco (Stanford University) for critical review of the manuscript. This work was supported by grants from the NIA to YW.C.F. (AG21068, AG31086).
References
- Baker KD, Thummel CS. Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab 2007; 6:257 - 266
- Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 2001; 292:107 - 110
- Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell 2005; 121:115 - 125
- Bauer JH, Chang C, Morris SN, Hozier S, Andersen S, Waitzman JS, et al. Expression of dominant-negative Dmp53 in the adult fly brain inhibits insulin signaling. Proc Natl Acad Sci USA 2007; 104:13355 - 13360
- Hwangbo DS, Gersham B, Tu MP, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature 2004; 429:562 - 566
- Baumeister R, Schaffitzel E, Hertweck M. Endocrine signaling in Caenorhabditis elegans controls stress response and longevity. J Endocrinol 2006; 190:191 - 202
- Hafen E. Cancer, type 2 diabetes and ageing: News from flies and worms. Swiss Med Wkly 2004; 134:711 - 719
- Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: Growth and diabetic phenotypes. Science 2002; 296:1118 - 1120
- Kim SK, Rulifson EJ. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature 2004; 431:316 - 320
- Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, Driege Y, et al. Longer lifespan, altered metabolism and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci USA 2005; 102:3105 - 3110
- Lee G, Park JH. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics 2004; 167:311 - 323
- Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr Biol 2002; 12:1293 - 1300
- Broughton SJ, Slack C, Alic N, Metaxakis A, Bass TM, Driege Y, et al. DILP-producing Median Neurosecretory Cells in the Drosophila Brain Mediate the Response of Lifespan to Nutrition. Aging Cell 2010; 9:336 - 346
- Fridell YW, Hoh M, Kreneisz O, Hosier S, Chang C, Scantling D, et al. Increased uncoupling protein (UCP) activity in Drosophila insulin-producing neurons attenuates insulin signaling and extends lifespan. Aging (Albany NY) 2009; 1:699 - 713
- Bouwman V, Adriaanse MC, van 't Riet E, Snoek FJ, Dekker JM, Nijpels G. Depression, anxiety and glucose metabolism in the general dutch population: The new Hoorn study. PLoS One 5:9971
- Guerra C, Navarro P, Valverde AM, Arribas M, Bruning J, Kozak LP, et al. Brown adipose tissue-specific insulin receptor knockout shows diabetic phenotype without insulin resistance. J Clin Invest 2001; 108:1205 - 1213
- Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science 2005; 307:384 - 387
- Fridell YW, Sanchez-Blanco A, Silvia BA, Helfand SL. Targeted expression of the human uncoupling protein 2 (hUCP2) to adult neurons extends life span in the fly. Cell Metab 2005; 1:145 - 152
- Erion DM, Shulman GI. Diacylglycerol-mediated insulin resistance. Nat Med 16:400 - 402
- Nakae J, Cao Y, Oki M, Orba Y, Sawa H, Kiyonari H, et al. Forkhead transcription factor FoxO1 in adipose tissue regulates energy storage and expenditure. Diabetes 2008; 57:563 - 576
- Belgacem YH, Martin JR. Disruption of insulin pathways alters trehalose level and abolishes sexual dimorphism in locomotor activity in Drosophila. J Neurobiol 2006; 66:19 - 32
- Nakae J, Oki M, Cao Y. The FoxO transcription factors and metabolic regulation. FEBS Lett 2008; 582:54 - 67
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, et al. Role of brain insulin receptor in control of body weight and reproduction. Science 2000; 289:2122 - 2125
- Staubli F, Jorgensen TJ, Cazzamali G, Williamson M, Lenz C, Sondergaard L, et al. Molecular identification of the insect adipokinetic hormone receptors. Proc Natl Acad Sci USA 2002; 99:3446 - 3451
- Schaffer MH, Noyes BE, Slaughter CA, Thorne GC, Gaskell SJ. The fruitfly Drosophila melanogaster contains a novel charged adipokinetic-hormone-family peptide. Biochem J 1990; 269:315 - 320
- Noyes BE, Katz FN, Schaffer MH. Identification and expression of the Drosophila adipokinetic hormone gene. Mol Cell Endocrinol 1995; 109:133 - 141
- Masternak MM, Panici JA, Wang F, Wang Z, Spong A. The effects of growth hormone (GH) treatment on GH and insulin/IGF-1 signaling in long-lived Ames dwarf mice. J Gerontol A Biol Sci Med Sci 65:24 - 30
- Zheng Y, Zhang W, Pendleton E, Leng S, Wu J, Chen R, et al. Improved insulin sensitivity by calorie restriction is associated with reduction of ERK and p70S6K activities in the liver of obese Zucker rats. J Endocrinol 2009; 203:337 - 347
- Zambon A, Hashimoto SI, Brunzell JD. Analysis of techniques to obtain plasma for measurement of levels of free fatty acids. J Lipid Res 1993; 34:1021 - 1028
- Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest 2002; 32:14 - 23
- Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 2007; 87:507 - 520
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 2001; 292:104 - 106
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 1997; 277:942 - 946
- Zhang H, Stallock JP, Ng JC, Reinhard C, Neufeld TP. Regulation of cellular growth by the Drosophila target of rapamycin dTOR. Genes Dev 2000; 14:2712 - 2724
- Rogina B, Wolverton T, Bross TG, Chen K, Muller HG, Carey JR. Distinct biological epochs in the reproductive life of female Drosophila melanogaster. Mech Ageing Dev 2007; 128:477 - 485
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature 1993; 366:461 - 464
- Wolkow CA, Kimura KD, Lee MS, Ruvkun G. Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science 2000; 290:147 - 150
- Accili D, Drago J, Lee EJ, Johnson MD, Cool MH, Salvatore P, et al. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat Genet 1996; 12:106 - 109
- Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 2003; 299:572 - 574