Abstract
The cohesin network has an essential role in chromosome segregation, but also plays a role in DNA damage repair. Eco1 is an acetyltransferase that targets subunits of the cohesin complex and is involved in both the chromosome segregation and DNA damage repair roles of the network. Using budding yeast as a model system, we find that mutations in Eco1, including a genocopy of a human Roberts syndrome allele, do not cause gross defects in chromosome cohesion. We examined how mitotic and meiotic DNA damage repair is affected by mutations in Eco1. Strains containing mutations in Eco1 are sensitive to DNA damaging agents that cause double-strand breaks, such as Xrays and bleomycin. While meiotic crossing over is relatively unaffected in strains containing the Roberts mutation, reciprocal mitotic crossovers occur with extremely low frequency in this mutant background. Our results suggest that Eco1 promotes the reciprocal exchange of chromosome arms and maintenance of heterozygosity during mitosis.
Introduction
Chromosome cohesion is essential to hold sister chromosomes together from the time they are replicated until the time they must be separated at the metaphase to anaphase transition. Cohesion is essential for the accurate attachment of chromosomes to the metaphase spindle and their subsequent division. Cohesion is mediated by the cohesin complex which consists of four subunits: Smc1, Smc3, Scc3 and Mcd1/Scc1/Rad21 (hereafter Mcd1). There are also several cohesin-associated proteins that are important for chromosome cohesion, including Eco1/Ctf7 (hereafter Eco1), an acetyltransferase important for the establishment of cohesion during S phase,Citation1 and Scc2-Scc4, a complex required to load the cohesin complex onto chromatin.Citation2
Mutation of human ESCO2, a human homolog of yeast ECO1, is associated with Roberts syndrome (RBS)Citation3 and mutation of SCC2/NIPBL, SMC1 and SMC3 have all been associated with Cornelia de Lange syndrome (CdLS).Citation4–Citation7 Together RBS and CdLS make up the cohesinopathies, which are human developmental disorders.Citation8,Citation9 Chromosome segregation defects are not a feature of these disorders. Instead, the molecular etiology is assumed to be related to non-essential functions of the cohesin network in gene regulation and DNA damage repair.Citation10
Along with chromosome cohesion, the cohesin network has been shown to be involved in chromosome condensation,Citation11,Citation12 gene regulation,Citation11,Citation13,Citation14 subnuclear chromatin organization,Citation11,Citation15 and double-strand break (DSB) repair.Citation16,Citation17 DSBs are a significant threat to genome stability and can arise from endogenous and exogenous sources such as collapsed replication forks and exposure to ionizing radiation. In most eukaryotes, DSBs that arise during S-phase or G2/M are repaired by homologous recombination (HR). HR repair of DNA damage requires an intact, homologous DNA sequence to serve as a template and during mitosis this is often the sister chromatid resulting in genetically silent repair.
When a DSB is induced by the budding yeast site-specific endonuclease HO in G2/M, cohesin binds to the region surrounding the breakCitation18,Citation19 in an Scc2-dependent manner.Citation20 Human cohesin has been shown to interact with Rad50 and accumulate in regions of laser-induced DNA damage during S and G2 phases in a Rad50/Mre11-dependent manner.Citation21 Furthermore, cohesion is reinforced genome-wide in G2/M in response to a single DSB. This process is dependent on the acetyltransferase activity of Eco1 and is referred to as damage-induced cohesion.Citation20,Citation22 Reinforcement of cohesion in response to DNA damage has also been shown to occur in human cells.Citation23 One target of Eco1 for damage-induced cohesion has been proposed to be Mcd1, although acetylation of Mcd1 has never been directly demonstrated in vivo.Citation24,Citation25 Acetylation of the cohesin subunit Smc3 has been shown to be critical for S phase coupled cohesion and is dependent on Eco1 in vivo.Citation26–Citation28 Acetylation of Smc3 by ESCO1, a human homolog of yeast ECO1, has also been shown to be important for the DNA damage response in human cells.Citation23 Additional Eco1 targets have been shown in vitroCitation29 but their biological significance is unknown.
Eco1 has two primary domains, a zinc finger in the N-terminus and an acetyltransferase domain in the C-terminus. The mutation W539G has been shown to abrogate acetyltransferase activity in the context of ESCO2,Citation30 a human ortholog of yeast ECO1, and this mutation is associated with RBS.Citation3 Cells from RBS patients are sensitive to the DNA damaging agents mitomycin C, camptothecin and etoposide, while no particular sensitivity to UV, ionizing radiation, hydroxyurea or aphidicolin was found.Citation30,Citation31 In budding yeast, mutations in the acetyltransferase domain have been shown to strongly reduce acetyltransferase activity in vitroCitation29 but do not cause high rates of chromosome loss.Citation32 In contrast, mutations in the zinc finger (C35Y, H53Y) have some acetyltransferase activity in vitro but still have very high rates of chromosome loss.Citation32 The zinc finger enhances the activity of Eco1 for cohesion, but is not essential for interaction with chromatin or protein targets.Citation33
DNA breaks caused by X-ray treatment persist in backgrounds containing mutations in the cohesin network,Citation17–Citation19 suggesting the damage is not efficiently repaired. Somewhat paradoxically, it has also been shown that damage-induced cohesion is not required for intrachromosomal gene conversion of an HO break or the 5′ to 3′ resection of this break.Citation18 Marker loss at the RDN1 locus, which is likely due to intrachromosomal or intersister recombination in a haploid, is similarly unaffected by mutation of the acetyltransferase domain of Eco1.Citation11 Thus, the exact molecular role of cohesion in DSB repair remains mysterious. One prevailing idea is that cohesin is involved in the process of selecting the sister as a template for repair, but this is unsupported by experimental evidence. The effect of cohesion on recombination between homologs has never been explored.
We examined how mutations in Eco1 affect interhomolog recombination in S. cerevisiae. We demonstrate that the W216G mutation is a phenocopy of the human RBS mutation in that it eliminates acetyltransferase activity in vitro. Using various measures such as in vivo acetylation of Smc3, precocious sister separation and DNA damage sensitivity, we compare four different mutations in Eco1. We then use the RBS mutant, which shows limited precocious sister separation but obvious damage sensitivity, to examine the effect on recombination. Following bleomycin treatment, we find a strong deficiency in reciprocal crossing over during mitotic growth, which is needed to maintain heterozygosity. In contrast, we find only subtle defects in meiotic recombination. These results imply that an Eco1-dependent process is needed for specific recombination outcomes, e.g., reciprocal mitotic crossing over, but not for others, such as break-induced replication, gene conversion or meiotic crossing over.
Results
Replication-coupled cohesion is mildly affected by mutations in Eco1.
We tested three different mutations in Eco1 for their effect on chromosome cohesion: (1) eco1-H53Y, which is expected to disrupt the zinc finger, (2) eco1-ack (R222G, K223G), which disrupts acetyltransferase activity and (3) eco1-W216G, which corresponds to the W539G mutation associated with Roberts syndrome (). Chromosome cohesion was examined in the Eco1 mutant strains in nocodazole-arrested cells using GFP marked telIVR (), an arm location on ChrIV () and cenIV ().Citation34 Nocodazole arrests were verified by cytometry. While the eco1-ack strain shows no defect in cohesion, the eco1-W216G mutant has a mild defect in cohesion and the eco1-H53Y mutant has a moderate defect in cohesion. The cohesion defect in the eco1-H53Y mutant is consistent with the report of elevated rates of chromosome loss in a zinc finger mutant.Citation32 However, this effect (15–20% precocious separation) is not as severe as other mutations that can cause as much as 80–90% loss of cohesion. At 37°C, the eco1-W216G strain shows 65% loss of cohesion.Citation11 The eco1-1 allele confers severe cohesion defects at 37°C, but also has cohesion defects (∼8% higher than WT at CenV) even at the “permissive” temperature of 22.5°C.Citation1 Because this mutant is very temperature sensitive, its phenotype is somewhat difficult to compare with eco1-ack, eco1-W216G and eco1-H53Y, which have a permissive temperature of 30°C.
Acetyltransferase activity is compromised by the eco1-W216G (RBS) mutation.
In order to compare the acetyltransferase activity of different Eco1 mutants, each mutant protein was expressed in E. coli and purified via a GST tag. Recombinant protein was incubated with 3H-acetyl-Co-A and a recombinant Mcd1 peptide (amino acids 169–337). As had been previously shown, the eco1-1 (G211D) and eco1-ack mutations strongly reduce both autoacetylation of Eco1 and acetylation of an exogenous substrate.Citation29 The Eco1-W216G mutant protein behaved similarly. In contrastt, the H53Y zinc finger mutation results in a protein that retains some auto-acetyltransferase activity, but has a similar deficiency to the other mutants in terms of acetylation of an exogenous substrate (). This protein might be expected to have low acetyltransferase activity toward its targets in vivo. Similar results were obtained when acetylation was detected by western blotting with an anti-acetyl-lysine antibody (data not shown). Thus, all four mutants have severely compromised acetyltransferase activity toward a target protein in vitro.
We next checked the expression of the mutants in vivo by adding a 3X FLAG tag to the C-terminus and immunoblotting. We find that Eco1-W216G and Eco1-H53Y are present at much lower levels than wild-type protein (12-fold and 6-fold, respectively, ). Regrettably, a strain bearing FLAG tagged Eco1-1 is inviable, so we were unable to measure the level of this mutant protein in vivo. The lower levels of the Eco1-W216G and Eco1-H53Y protein in vivo combined with the lack of acetyltransferase activity measured in vitro suggest these mutants might have a stronger phenotype than eco1-ack, whose levels are only reduced ∼2-fold.
Given the differences in protein levels, we decided to measure the acetylation levels of Smc3 in each mutant background in vivo in order to determine their “true” acetylation defect. Acetylation of Smc3 can be detected with an anti-acetylysine antibody when the cohesin complex is immunoprecipitated from whole cell extracts. We examined Smc3 acetylation using immunoprecipitation of either Smc3-HA or Mcd1-18Myc. Smc3-HA in combination with the eco1-H53Y mutation is lethal so we could not perform the HA immunoprecipitation in this strain. We found that the level of Smc3 acetylation in eco1-ack is nearly wild-type, while the level in the eco1-1 is the lowest. Acetylation is present at intermediate levels in the eco1-H53Y and eco1-W216G mutants (). The level of acetylation measured in either the Mcd1 or Smc3 pull-down is similar. In addition, each of the pull-downs was performed at least twice with similar results. Unfortunately, the level of acetylated Mcd1 cannot be measured in vivo since it is not detected with any of the available anti-acetyl-lysine antibodies.Citation24 Although acetylation of an exogenous substrate is undetectable in vitro, these mutant Eco1 proteins mediate various levels of acetylation in vivo.
DNA damaging agents reduce the growth of strains with mutations in Eco1.
Given the role of the cohesin network in DNA repair, we examined the different mutants for damage sensitivity. The scc2-D730V mutant strain used in this assay corresponds to a mutation associated with a second cohesinopathy, Cornelia de Lange syndrome. Since both the eco1-W216G and scc2-D730V mutations have been reported to affect chromosome compaction, the scc2-D730V mutant is meant to serve as a control for any effect of chromosome compaction on damage sensitivity. The scc2-D730V mutation does not cause a measurable cohesion defect.Citation11 A rad50Δ mutant serves as a radiation sensitive control. Deletion of RAD61/WPL1 has previously been shown to rescue the growth of eco1-1 at 37°C and damage sensitivity associated with the eco1-1 mutation.Citation27
We examined the sensitivity of the eco1 mutants to different DNA damaging agents. Hydroxyurea (HU) will slow S phase which in turn causes collapsed and stalled replication forks. X-rays and bleomycin will primarily cause DSBs, which can occur at any point in the cell cycle. The eco1-1 mutant strain is sensitive to HU, bleomycin and X-rays. We find that none of the other 3 eco1 mutants nor the scc2 mutant display sensitivity to HU, but eco1-H53Y and eco1-W216G are both sensitive to X-rays and bleomycin (). The D730V mutation in SCC2 does not cause sensitivity to DNA damaging agents. As previously shown, the eco1-W216G mutant does not grow at 37°C,Citation11 but growth is rescued by deletion of RAD61. In contrast to what has been reported for eco1-1,Citation27 deletion of RAD61 does not rescue the damage sensitivity of the eco1-W216G strain. Deletion of RAD61 alone causes very mild DNA damage sensitivity, consistent with a previous report.Citation27 Similar results for all mutants were observed in an S288C background (data not shown).
In order to pinpoint the relevant acetylation target of Eco1 for damage sensitivity, we tried to genetically rescue the damage sensitivity by overexpression of known targets. Acetylation of Mcd1 is thought to be important for cohesion following a DSB in G2/M,Citation25 although this post-translational modification has never been detected in vivo. Expression of Mcd1 with mutations that mimic acetylation (K84Q, K210Q) bypassed the need for Eco1 for DSB-induced cohesion.Citation24 Smc3 has also been shown to be acetylated by Eco1 for S phase cohesion on residues K112 and K113.Citation26–Citation28,Citation35 We investigated whether overexpression of MCD1, MCD1K84QK210Q, SMC3, SMC3K112QK113Q, SMC3K112NK113N, SMC3K113Q or SMC3K113N would rescue the damage sensitivity of the eco1-W216G mutant strain. We found that overexpression of MCD1 or MCD1K84QK210Q did not suppress the growth defect caused by X-rays (). Neither didt overexpression of SMC3 or any of the SMC3 mutants (). In conclusion, overexpression of no single known target of Eco1 was able to rescue the damage sensitivity of the eco1-W216G mutant strain. This result suggests that the expression of the acetylation mimic mutations may not sufficiently mimic the acetylated state or that Eco1 may have additional targets for DNA damage repair.
In contrast to the DNA damage sensitivity, overexpression of SMC3K113Q or SMC3K113N, but not SMC3, rescued the temperature sensitivity of the eco1-W216G strain. The rescue suggests that the primary reason for lethality at 37°C, which is correlated with a strong cohesion defect,Citation11 is a deficit in acetylation of K113 in Smc3 by Eco1. These findings echo the rescue of the eco1-1 strain with SMC3K113N.Citation27 Overexpression of SMC3K112NK113N or SMC3K112QK113Q actually inhibited growth of the eco1-W216G strain, suggesting that the constituitive acetylation of both residues is problematic, similar to findings in a previous report.Citation26
As a control, we overexpressed ECO1, which allows robust growth following treatment with X-rays, as expected. Unexpectedly, overexpression of Eco1-W216G rescued the temperature sensitivity of the eco1-W216G mutant strain (), suggesting an abundance of Eco1-W216G is sufficient for its essential function in chromosome segregation, but in contrast, it did not suppress the damage sensitivity. Western blot analysis showed that the eco1-W216G mutant protein is expressed at high levels () and furthermore, that the acetylation of Smc3 is restored by the overexpression (). Thus, neither the restoration of acetylated Smc3 nor the expression of the Smc3 acetylation mimic is able to rescue the damage sensitivity of the eco1-W216G mutant.
Eco1 mutants activate the DNA damage checkpoint.
In order to further characterize the DNA damage defect in the Eco1 mutants, we analyzed checkpoint function in the mutants. The sensitivity of eco1 mutants to DNA damage may result from either a failure activate the DNA damage checkpoint or an inability to repair the damage. In budding yeast the effector kinase, Rad53 (Chk2) is required for checkpoint activity and cell cycle arrest in response to DNA damage.Citation36 Rad53 becomes phosphorylated by Mec1 in a Rad9-dependent manner in response to DNA double strand breaks.Citation37 We monitored cell cycle progression and the phosphorylation state of Rad53 following exposure of cells to X-rays ().
Rad53 was efficiently phosphorylated following treatment with X-rays in the eco1-H53Y, eco1-W216G and eco1-ack mutants, but only the eco1-H53Y and eco1-W216G mutants held a persistent arrest in G2/M, similar to a rad50Δ mutant (). In contrast, the eco1-ack mutant behaved more like WT; it did not arrest, suggesting it could efficiently correct the damage. This observation supports our finding that eco1-H53Y and eco1-W216G mutant strains are more sensitive to genotoxic stress than eco1-ack (). A rad9Δ mutant serves as a control; Rad53 is not phosphorylated in this mutant and cells do not arrest.Citation38,Citation39 We conclude that the checkpoint is appropriately activated in the mutants and the sensitivity to ionizing radiation is due to a downstream defect. This observation is consistent with damage-induced cohesion being dependent on Mec1.Citation22
Reciprocal mitotic crossovers require the acetyltransferase activity of Eco1.
While mutations that reduce the acetyltransferase activity of Eco1 have little effect on S phase cohesion and chromosome segregation, the same mutations (eco1-ack, eco1-203) have been demonstrated to reduce DSB-coupled cohesion in G2/M.Citation22 Unfortunately the eco1-W216G mutation in combination with the mcd1-1 allele used in the assay developed by the Koshland lab to measure damage-induced cohesion is a lethal combination so we cannot measure damage-induced cohesion in this manner. However, since the Roberts strain is damage sensitive, we decided to study how this mutation affects recombination outcomes in a diploid background.
An elegant system has been developed to analyze mitotic recombination in a 120 kb interval on chromosome V.Citation40 This system takes advantage of a variety of heteroallelic selectable markers. One copy of chromosome V contains HIS3, can1–100 and HYG while the second copy of chromosome V contains LEU2, SUP4-o (an ochre suppressor) and KAN at the same positions. The background is homozygous for the ade2-1 allele, which contains an ochre suppressible mutation. When ade2-1 is suppressed by SUP4-o, cells are white, but if the cells lack SUP4-o, they will be red (). This strain can be used to score break induced replication (BIR), local gene conversion (GC), reciprocal crossovers (RCO) and chromosome loss events.
Previously, mitotic recombination following growth on HU was examined.Citation40 HU increases the number of recombinants approximately 40-fold over the rate of spontaneous mitotic recombination. When the rate of recombination is high, recombination events do not need to be selected. The number of sectored colonies relative to the total number of colonies is a direct measure of the frequency of recombination. After the two colored sectors are isolated, these colonies can be further scored for the various markers to differentiate types of recombination events.
We grew the diploid WT and eco1-W216G mutant strains on plates containing 2.5 µg/ml bleomycin to induce a low level of damage. At this concentration, the eco1-W216G mutant strain can still grow whereas at higher levels, its growth is significantly impaired (). Individual colonies were resuspended in water and plated to nonselective medium (SD-arg) and screened for colonies with red/white sectors. We find that growth on 2.5 ug/ml bleomycin elevates the rate of recombination in a WT strain approximately 120-fold compared to no treatment. Both RCO and BIR events are stimulated on bleomycin in a WT strain. However, with HU the ratio of RCO to BIR events is approximately 0.6:1,Citation40 while growth on bleomycin causes RCOs to become more common than BIR events with a shift in the ratio to 1.8:1. This is likely related to the different types of damage caused by HU and bleomycin. The overall rate of recombination in the eco1-W216G background appears slightly lower than wild-type (p = 0.08, chi square test, ), suggesting the mutation may compromise recombination efficiency.
Sectored colonies were scored to assess what type of recombination event had occurred. For the WT strain we examined 11,340 total colonies and found 18 with the phenotype expected for RCO events and 10 with the phenotype expected for BIR events. For the eco1-W216G mutant strain, we examined 24,489 total colonies and found 6 with the phenotype expected for an RCO event and 32 with the phenotype expected for a BIR event. The drop in RCO events in the mutant background is statistically significant at the level of p < 0.0001 using a chi square test with Yates correction. Three independent trials were performed and the standard deviation is shown (). Chromosome loss events were not significantly elevated in the Roberts mutant background, arguing that chromosome segregation is not defective and consistent with the 1 spot-2 spot results (). The specific effect of the eco1-W216G mutation on reciprocal crossovers implies that Eco1 activity is needed for this particular recombination outcome in mitotic cells. Interestingly RCOs seem to be particularly important for mitotic repair at the rDNA.Citation41
Eco1 activity is not essential for meiotic crossovers.
The reduction in reciprocal crossovers during mitosis prompted us to examine the level of crossovers in meiosis in the Roberts mutant background. In meiosis, programmed DSBs elevate the frequency of recombination 1,000-fold. In addition to the markers already described, the strain used was heterozygous for URA3/ura3-1 within the 120 kb interval on ChrV and for TRP1 inserted at the TAR1 locus within the rDNA (). We wanted to monitor recombination within RDN1 since this is a cohesin binding site.Citation42,Citation43 In mitosis, the cohesin complex consists of Smc1, Smc3, Scc3 and Mcd1/Scc1. In meiosis, there is a second cohesin complex in which Rec8 replaces Mcd1.Citation16,Citation44 In contrast to Mcd1, Rec8 cannot mediate damage-induced cohesion.Citation25 Whether or not Eco1 has essential targets in meiosis is an open question.
We examined the meiotic products from a WT strain and a Roberts mutant strain. Tetrad formation is reduced in the Roberts mutant relative to the wild-type. Furthermore, spore viability is reduced from 96 to 75%. However, recombination as scored in 4-spore tetrads is indistinguishable from WT. Although the frequency of aberrant segregation for four different markers is elevated in the mutant, these increases are not statistically significant (). If aberrant segregation is summed for all the loci, the increase becomes statistically significant with a p value of 0.012. Genetic distance, which is a measure of the number of crossovers per kb, is similar for three different intervals (). Thus, it seems that Eco1 activity is not essential for successful meiotic recombination or crossing over.
Discussion
We have analyzed and compared four mutations in the essential acetyltransferase ECO1; each mutant has a distinct phenotype. Together these mutations can be thought of as a series of weak to strong hypomorphic mutations. Three mutations are in the acetyltransferase domain and one is in the zinc finger; all four mutations cause deficiencies in acetyltransferase activity in vitro, including a mutation that corresponds to the human Roberts syndrome mutation. Despite similar deficiencies in acetyltransferase activity in vitro, strains with the mutations integrated under the control of the endogenous promoter display different levels of Smc3 acetylation in vivo and these levels roughly correlate with sensitivity to agents that cause DNA damage, with the eco1-1 mutant being the most sensitive, eco1-W216G and eco1-H53Y displaying intermediate sensitivity and eco1-ack showing no detectable sensitivity. Since acetylation of Smc3 is necessary for cohesion, the number of cohesive complexes is likely to be reduced in the mutants. Reducing acetylated complexes may be akin to systematically reducing Mcd1 levels, which has recently been argued to reduce the number of cohesive complexes.Citation45 In this case, accurate chromosome segregation required much less Mcd1 than DNA repair,Citation45 which is consistent with our findings that reduced Smc3 acetylation in the eco1-W216G mutant does not affect chromosome segregation but this mutation does affect DNA repair.
Growth at 37°C in the eco1-W216G strain was rescued by overexpression of ECO1-W216G, SMC3K113Q, SMC3K113N or deletion of RAD61, suggesting these genetic manipulations are sufficient for chromosome segregation. In contrast, none of these genetic manipulations was able to rescue the damage sensitivity, suggesting none of these genetic states are sufficient for an effective response to DNA damage. It is possible that the overexpression of ECO1-W216G, SMC3K113Q or SMC3K113N failed to rescue the damage sensitivity because acetylation is uncoupled with the damage response.
Given the DNA damage sensitivity of the eco1-W216G strain, we undertook a detailed characterization of recombination in this mutant background. The mutant displayed a surprising defect in reciprocal crossing over, suggesting that an Eco1-dependent process is required for the exchange of homologous chromosome arms. This defect may be due to a reduction in damage-induced cohesion. The specificity of the effect for mitotic recombination probably reflects the different roles of cohesin in the repair of mitotic DSBs and the programmed DSBs that occur during meiosis.
When DSBs are repaired by non-reciprocal recombination, such as BIR, instead of reciprocal crossovers (RCO), such as in the RBS mutant strain, the net result is a greater chance for loss of heterozygosity (LOH). Although crossing over is a rather rare event during mitosis, mechanisms to ensure reciprocity are quite important since they allow for the maintenance of two copies of a given allele, rather than a conversion to homozygosity. Retinoblastoma, a human cancer of the retina, provides a compelling example for the importance of maintenance of heterozygosity. When one parent's contribution of the tumor suppressor Rb1 is flawed, this almost invariably results in childhood retinoblastoma due to chance LOH events. Thus, discovering mutations that lead to a bias toward LOH is informative since it identifies a potential mechanism by which LOH can occur. In budding yeast that have been aged by multiple passages, the rate of LOH increases due to a bias toward non-reciprocal recombination,Citation46 similar to what we observe in the RBS strain.
Materials and Methods
GST-fusion protein purification.
An E. coli culture was grown in 1 L of LB with 100 µg/ml ampicillin at 37°C until OD600 was 0.3. Then the culture was shifted to 25°C and continued to grow until OD600 was 0.6. IPTG was added to 0.3 mM for 3 hours at 25°C. The cells were subjected to centrifugation at 5 K, 4°C for 5 min and washed with cold PBS. The pellet was resuspended in 30 ml metal lysis buffer (25 mM Hepes, pH 7.5, 300 mM NaCl, 10% glycerol and 1 mM DTT) with protease inhibitors (Roche). Then 0.5 ml of 500 mg/ml lysozyme was added and mixed by inverting. The mixture was incubated 10 minutes on ice and sonicated on ice 5 times for 30 seconds on 35% power with a 2 minute interval. The lysate was then centrifuged for 20 minutes 16 K at 4°C and was then further centrifuged at 50 K for 45 minutes. The supernatant was added to glutathione resin and rotated for 2 hours at 4°C. The glutathione resin was rinsed with 5 ml metal lysis buffer and protein was eluted by adding 1 ml of 10 mM reduced glutathione.
In vitro acetylation assay.
Purified GST-tagged Eco1p WT, G211D, R222G K223G, W216G and H53Y proteins (0.4 µg) were added to HAT buffer (50 mM Tris [pH 8.0], 5% glycerol, 0.1 mM EDTA, 50 mM KCl, 1 mM DTT, 1 mM PMSF and 10 µM 3H-Acetyl-CoA) and incubated with GST-Mcd1169–337 (1 µg) for 60 minutes at 30°C. Reactions were run on SDS-PAGE and analyzed by both Coomassie staining and exposure to a Phosphor screen.
α-FLAG, α-Eco1, α-Rad53 and α-acetyl-lysine western blots.
The cell pellet from a 200 ml culture grown to OD600 = 0.8 in YPD was lysed in NP40 Lysis Buffer (50 mM Tris pH 7.5, 150 mM NaCl, 10% Glycerol, 1% NP-40) by the addition of glass beads and beadbeating for 60 sec 5 times with 2 min intervals on ice. The supernatant was collected and the protein concentration was determined using the optical density of a standard curve and Bradford reagent. 50 µg of total protein was loaded onto a 4–12% Bis-Tris gel for SDS-PAGE. Proteins were transferred to a nitrocellulose membrane followed by blocking. For detection of Eco1-FLAG, α-FLAG antibody (Sigma #F3165) was used at a 1:2,500 concentration followed by α-Mouse HRP (GE Healthcare #NA931V) at a 1:5,000 concentration. For detection of overexpressed Eco1, polyclonal α-Eco1 antibody was used at 1:1,000 followed by α-rabbit-HRP (GE Healthcare #NA934V) at a 1:5,000 concentration. Smc3-HA was pulled down from whole cell extracts using anti-HA beads (Roche) and eluted using SDS buffer (10 mM Tris pH 7.5, 1 mM EDTA, 1% SDS). A cocktail of deacetylase inhibitors (100 µM Trichostatin A, 50 mM nicotinamide, 50 mM sodium butyrate) was added during the immunoprecipitation process. The eluate was subjected to SDS-PAGE. Following transfer to a nitrocellulose membrane and blocking, α-acetyl-lysine antibody (Cell Signaling #9441) was used at a 1:1,000 concentration followed by α-Rabbit HRP as above. Detection was achieved with Amersham ECL Plus western Blotting Detection System (GE Healthcare #RPN2132).
To detect phosphorylated Rad53, 75 µgs of protein were subjected to SDS-PAGE. Following transfer to a nitrocellulose membrane and blocking, α-phosphoRad53 antibody (Santa Cruz, SC-6749) was used at a concentration of 1:1,000 followed by α-goat-AP (Promega, V1151) at a 1:5,000 concentration. For signal detection, BCIP (5-bromo-4-chloro-3-indolyl-phosphate) was used in conjunction with NBT (nitro blue tetrazolium) for the colorimetric detection of alkaline phosphatase activity in the presence of alkaline phosphatase buffer [100 mM Tris-HCl [pH 9.0], 150 mM NaCl, 1 mM MgCl2].
Yeast strains () and tetrad analysis were constructed and analyzed by standard methods.
The cohesion assays were carried out as previously described.Citation11
The assay to select and analyze reciprocal mitotic crossovers has been extensively described.Citation40
Summary
The cohesin complex ties sister chromatids together from the time they are copied until they are separated into two new cells. Cohesin is evolutionarily conserved from yeast to man. While cohesin is critical for chromosome segregation, it also plays a role in DNA double-strand break repair. Eco1, an acetyltransferase, regulates the complex. We used budding yeast to study how mutations in ECO1 affect the DNA damage response. A mutation in ECO1 associated with Roberts syndrome caused a deficit in the reciprocal exchange of homologous chromosome arms. This type of recombination outcome is considered beneficial since it promotes heterozygosity of alleles.
Figures and Tables
Figure 1 Cohesion defects in Eco1 mutant strains. (A) The two domains of Eco1 are shown in a cartoon of the gene and the mutations characterized are indicated. (B) Yeast strains were constructed by replacing the genomic copy of the indicated allele with an Eco1 mutant cohesinopathy allele, thereby placing the mutant allele under the control of the endogenous promoter at the endogenous locus. Additionally, strains contained lacO repeats on the telomere (B), arm (C) or centromere (D) of chromosome IV and a lacI-GFP fusion was inducibly expressed in nocodazole-arrested cells at 30°C. The arrest was confirmed by cytometry (data not shown). The number of cells displaying 1 spot versus 2 spots was counted to determine chromosome cohesion. At least three biological replicates were conducted for each strain and at least 300 total cells were counted. The standard deviation is indicated. Statistical significance was calculated using Fisher's exact test.
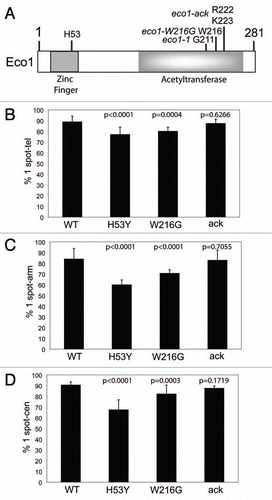
Figure 2 Acetyltransferase activity associated with Eco1 mutants. GST-Eco1 and GST-Mcd1169–337 fusion proteins were expressed in E. coli and purified by glutathione-agarose. Following an in vitro acetylation reaction with 3H-acetylCoA in which Mcd1 peptide was either included (+) or omitted (−), the sample was subjected to autoradiography or Coomassie staining following PAGE. Eco1 can autoacetylate (upper band) and can also acetylate Mcd1169–337 (lower band). (B) The expression of Eco1 was measured by immunoblotting with α-FLAG antibody in whole cell yeast extracts. A 3XFLAG tag was integrated at the C-terminus of each protein. 50 µg of total protein was loaded per lane. The expression level of each protein is shown normalized to wild-type. The asterisk indicates a non-specific band. G6PDH serves as a loading control. (C) The level of acetylation of Smc3 was measured in WT, eco1-1, eco1-W216G, eco1-H53Y and eco1-ack mutant strains by pulling down either Smc3-HA with anti-HA beads or Mcd1-18Myc with anti-Myc beads from whole cell extracts followed by western blotting with anti-acetyl-lysine antibody.
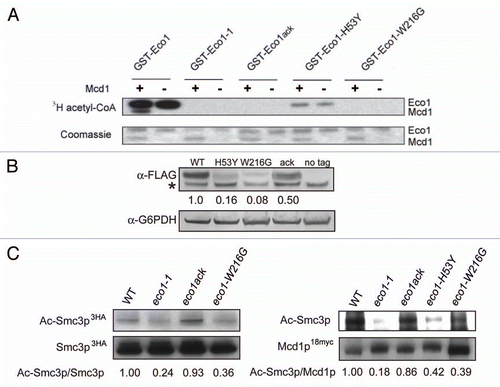
Figure 3 Eco1 mutants are sensitive to agents that cause DNA double-strand breaks. Haploid strains of the indicated genotype were serially diluted, plated and grown on SD complete medium at 24°C, 30°C or 37°C, subjected to 75 Gray of ionizing radiation followed by growth at 30°C or plated onto SD complete medium containing 50 or 100 mM hydroxyurea (HU) or 5 or 10 µg/ml bleomycin, followed by growth at 30°C.
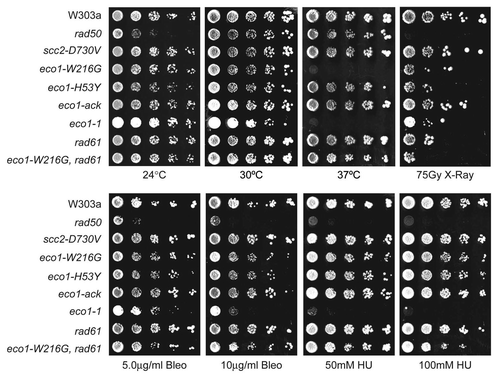
Figure 4 Overexpression of known targets of Eco1 does not suppress the damage sensitivity of the eco1-W216G strain. An eco1-W216G and Wt strain was transformed with plasmids containing various genes under the control of a gal promoter or an empty vector (EV). Strains were plated onto galura medium to induce expression and incubated at 30°C or treated with 37.5 Gray of ionizing radiation followed by incubation at 30°C or incubated at 37°C. (B) Strains with plasmids containing ECO1, eco1-W216G or an empty vector were grown in galactose to examine levels of Eco1 protein using anti-Eco1 antibody by western blot. Pgk1 serves as a loading control. “ns” indicated a non-specific band. (C) From strains containing the plasmids in (B), an IP was performed with anti-HA antibody to pull down Smc3, and subsequently subjected to western blotting with anti-acetyl-lysine antibody. The level of Smc3 acetylation was quantitated using the level of Smc3 to normalize for IP efficiency and the numbers are shown below each lane.
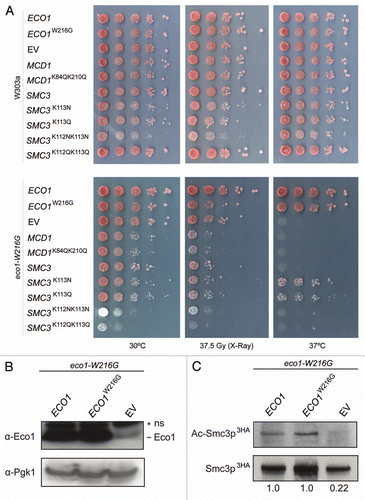
Figure 5 DNA damage checkpoint response in eco1 mutants. (A) Strains were grown to an OD600 of 0.6 and subjected to 75 Gray of ionizing radiation. Following exposure, cells were plated to YPD plates to measure viability. (B) Cells were collected for analysis of DNA content by cytometry and phosphorylation of Rad53 by western blotting prior to exposure (0) and at 2 and 8 hours following exposure.
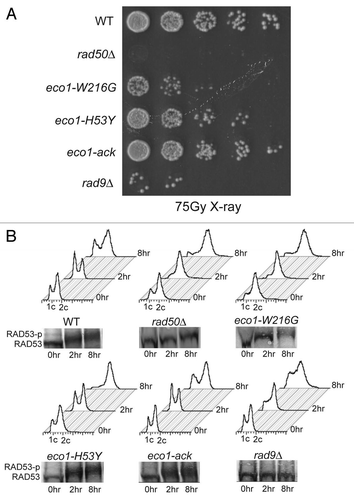
Figure 6 A genetic system to detect mitotic recombination events was used to score recombination in the Roberts mutant. (A) Schematic of the markers contained on Chromosome V. (B) Diploid strains were serially diluted and plated. The eco1-W216G diploid strains show similar sensitivity to bleomycin and ionizing radiation as the haploid strain (). (C) A wild-type strain and two independent isolates of the Roberts mutant strain were grown at room temperature on plates containing 2.5 g/µl bleomycin. Individual colonies were picked and plated onto SD-arg medium. Sectored colonies were scored as previously described.Citation40 “Total rate” indicates the total number of recombination events. These events were further broken down into reciprocal crossovers (RCO), break induced replication (BIR), local gene conversion (GC) and chromosome loss (Chr loss) based on marker scoring. The results are shown for each of the two mutant strains separately and combined (av). Three independent experiments were performed for each strain and the standard deviation is shown. The p value is derived from a chi square test comparing total recombinants—RCOs and RCOs from the WT strain to the sum of the same values from the eco1-W216G mutant strains.
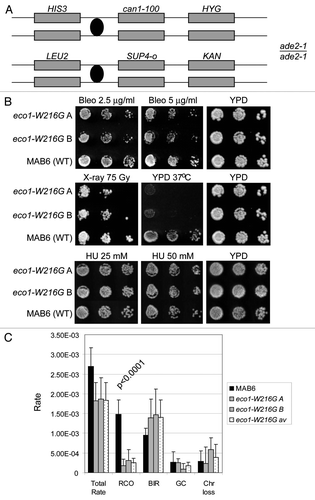
Figure 7 A genetic system to score meiotic recombination was used to analyze recombination in the Roberts mutant. (A) Schematic of the markers contained on Chromosome V. In addition, TRP1 was inserted into the RDN1 repeat in one parent. (B) Aberrant segregation (exceptions to 2:2 segregation) of markers was scored for four different positions in 305 wild-type and 588 mutant tetrads. Two independently isolated strains were used for each genotype. Fisher's exact test indicates no significant differences between mutant and wild-type. (C) The genetic distance was calculated for the three intervals indicated, with the caveat that pink/white color was used to score the SUP4-o locus. Fisher's exact test indicates no significant differences between mutant and wild.
Table 1 Strains
Acknowledgements
We thank Doug Koshland for strains, and Tom Petes for strains, advice and critical reading of the manuscript. We thank Mark Mattingly, Kenny Lee, Jen Gardner and Bethany Harris for technical assistance.
References
- Toth A, Ciosk R, Uhlmann F, Galova M, Schleiffer A, Nasmyth K. Yeast cohesin complex requires a conserved protein, Eco1p(Ctf7), to establish cohesion between sister chromatids during DNA replication. Genes Dev 1999; 13:320 - 333
- Ciosk R, Shirayama M, Shevchenko A, Tanaka T, Toth A, Nasmyth K. Cohesin's binding to chromosomes depends on a separate complex consisting of Scc2 and Scc4 proteins. Mol Cell 2000; 5:243 - 254
- Vega H, Waisfisz Q, Gordillo M, Sakai N, Yanagihara I, Yamada M, et al. Roberts syndrome is caused by mutations in ESCO2, a human homolog of yeast ECO1 that is essential for the establishment of sister chromatid cohesion. Nat Genet 2005; 37:468 - 470
- Deardorff MA, Kaur M, Yaeger D, Rampuria A, Korolev S, Pie J, et al. Mutations in cohesin complex members SMC3 and SMC1A cause a mild variant of cornelia de Lange syndrome with predominant mental retardation. Am J Hum Genet 2007; 80:485 - 494
- Musio A, Selicorni A, Focarelli ML, Gervasini C, Milani D, Russo S, et al. X-linked Cornelia de Lange syndrome owing to SMC1L1 mutations. Nat Genet 2006; 38:528 - 530
- Krantz ID, McCallum J, DeScipio C, Kaur M, Gillis LA, Yaeger D, et al. Cornelia de Lange syndrome is caused by mutations in NIPBL, the human homolog of Drosophila melanogaster Nipped-B. Nat Genet 2004; 36:631 - 635
- Tonkin ET, Wang TJ, Lisgo S, Bamshad MJ, Strachan T. NIPBL, encoding a homolog of fungal Scc2-type sister chromatid cohesion proteins and fly Nipped-B, is mutated in Cornelia de Lange syndrome. Nat Genet 2004; 36:636 - 641
- McNairn AJ, Gerton JL. Cohesinopathies: One ring, many obligations. Mutat Res 2008; 647:103 - 111
- Liu J, Krantz ID. Cohesin and human disease. Annu Rev Genomics Hum Genet 2008; 9:303 - 320
- McNairn AJ, Gerton JL. The chromosome glue gets a little stickier. Trends Genet 2008; 24:382 - 389
- Gard S, Light W, Xiong B, Bose T, McNairn AJ, Harris B, et al. Cohesinopathy mutations disrupt the subnuclear organization of chromatin. J Cell Biol 2009; 187:455 - 462
- Guacci V, Koshland D, Strunnikov A. A direct link between sister chromatid cohesion and chromosome condensation revealed through the analysis of MCD1 in S. cerevisiae. Cell 1997; 91:47 - 57
- Donze D, Adams CR, Rine J, Kamakaka RT. The boundaries of the silenced HMR domain in Saccharomyces cerevisiae. Genes Dev 1999; 13:698 - 708
- Rollins RA, Morcillo P, Dorsett D. Nipped-B, a Drosophila homologue of chromosomal adherins, participates in activation by remote enhancers in the cut and Ultrabithorax genes. Genetics 1999; 152:577 - 593
- Adelfalk C, Janschek J, Revenkova E, Blei C, Liebe B, Gob E, et al. Cohesin SMC1beta protects telomeres in meiocytes. J Cell Biol 2009; 187:185 - 199
- Klein F, Mahr P, Galova M, Buonomo SB, Michaelis C, Nairz K, et al. A central role for cohesins in sister chromatid cohesion, formation of axial elements and recombination during yeast meiosis. Cell 1999; 98:91 - 103
- Sjogren C, Nasmyth K. Sister chromatid cohesion is required for postreplicative double-strand break repair in Saccharomyces cerevisiae. Curr Biol 2001; 11:991 - 995
- Unal E, Arbel-Eden A, Sattler U, Shroff R, Lichten M, Haber JE, et al. DNA damage response pathway uses histone modification to assemble a double-strand breakspecific cohesin domain. Mol Cell 2004; 16:991 - 1002
- Strom L, Lindroos HB, Shirahige K, Sjogren C. Postreplicative recruitment of cohesin to double-strand breaks is required for DNA repair. Mol Cell 2004; 16:1003 - 1015
- Strom L, Karlsson C, Lindroos HB, Wedahl S, Katou Y, Shirahige K, et al. Postreplicative formation of cohesion is required for repair and induced by a single DNA break. Science 2007; 317:242 - 245
- Kim ST, Xu B, Kastan MB. Involvement of the cohesin protein, Smc1, in Atm-dependent and independent responses to DNA damage. Genes Dev 2002; 16:560 - 570
- Unal E, Heidinger-Pauli JM, Koshland D. DNA double-strand breaks trigger genome-wide sister-chromatid cohesion through Eco1 (Ctf7). Science 2007; 317:245 - 248
- Kim BJ, Li Y, Zhang J, Xi Y, Yang T, Jung SY, et al. Genome-wide reinforcement of cohesin binding at preexisting cohesin sites in response to ionizing radiation in human cells. J Biol Chem 2010; In press
- Heidinger-Pauli JM, Unal E, Koshland D. Distinct targets of the Eco1 acetyltransferase modulate cohesion in S phase and in response to DNA damage. Mol Cell 2009; 34:311 - 321
- Heidinger-Pauli JM, Unal E, Guacci V, Koshland D. The kleisin subunit of cohesin dictates damage-induced cohesion. Mol Cell 2008; 31:47 - 56
- Unal E, Heidinger-Pauli JM, Kim W, Guacci V, Onn I, Gygi SP, et al. A molecular determinant for the establishment of sister chromatid cohesion. Science 2008; 321:566 - 569
- Ben-Shahar TR, Heeger S, Lehane C, East P, Flynn H, Skehel M, et al. Eco1-dependent cohesin acetylation during establishment of sister chromatid cohesion. Science 2008; 321:563 - 566
- Zhang J, Shi X, Li Y, Kim BJ, Jia J, Huang Z, et al. Acetylation of Smc3 by Eco1 is required for S phase sister chromatid cohesion in both human and yeast. Mol Cell 2008; 31:143 - 151
- Ivanov D, Schleiffer A, Eisenhaber F, Mechtler K, Haering CH, Nasmyth K. Eco1 is a novel acetyltransferase that can acetylate proteins involved in cohesion. Curr Biol 2002; 12:323 - 328
- Gordillo M, Vega H, Trainer AH, Hou F, Sakai N, Luque R, et al. The molecular mechanism underlying Roberts syndrome involves loss of ESCO2 acetyltransferase activity. Hum Mol Genet 2008; 17:2172 - 2180
- van der Lelij P, Godthelp BC, van Zon W, van Gosliga D, Oostra AB, Steltenpool J, et al. The cellular phenotype of Roberts syndrome fibroblasts as revealed by ectopic expression of ESCO2. PLoS ONE 2009; 4:6936
- Brands A, Skibbens RV. Ctf7p/Eco1p exhibits acetyltransferase activity—but does it matter?. Curr Biol 2005; 15:50 - 51
- Onn I, Guacci V, Koshland DE. The zinc finger of Eco1 enhances its acetyltransferase activity during sister chromatid cohesion. Nucleic Acids Res 2009; 37:6126 - 6134
- Straight AF, Belmont AS, Robinett CC, Murray AW. GFP tagging of budding yeast chromosomes reveals that protein-protein interactions can mediate sister chromatid cohesion. Curr Biol 1996; 6:1599 - 1608
- Rowland BD, Roig MB, Nishino T, Kurze A, Uluocak P, Mishra A, et al. Building sister chromatid cohesion: smc3 acetylation counteracts an antiestablishment activity. Mol Cell 2009; 33:763 - 774
- Weinert TA, Kiser GL, Hartwell LH. Mitotic checkpoint genes in budding yeast and the dependence of mitosis on DNA replication and repair. Genes Dev 1994; 8:652 - 665
- Sanchez Y, Desany BA, Jones WJ, Liu Q, Wang B, Elledge SJ. Regulation of RAD53 by the ATM-like kinases MEC1 and TEL1 in yeast cell cycle checkpoint pathways. Science 1996; 271:357 - 360
- Weinert TA, Hartwell LH. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science 1988; 241:317 - 322
- de la Torre-Ruiz MA, Green CM, Lowndes NF. RAD9 and RAD24 define two additive, interacting branches of the DNA damage checkpoint pathway in budding yeast normally required for Rad53 modification and activation. EMBO J 1998; 17:2687 - 2698
- Barbera MA, Petes TD. Selection and analysis of spontaneous reciprocal mitotic cross-overs in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 2006; 103:12819 - 12824
- Casper AM, Mieczkowski PA, Gawel M, Petes TD. Low levels of DNA polymerase alpha induce mitotic and meiotic instability in the ribosomal DNA gene cluster of Saccharomyces cerevisiae. PLoS Genet 2008; 4:1000105
- Laloraya S, Guacci V, Koshland D. Chromosomal addresses of the cohesin component Mcd1p. J Cell Biol 2000; 151:1047 - 1056
- Glynn EF, Megee PC, Yu HG, Mistrot C, Unal E, Koshland DE, et al. Genome-wide mapping of the cohesin complex in the yeast Saccharomyces cerevisiae. PLoS Biol 2004; 2:259
- Watanabe Y, Nurse P. Cohesin Rec8 is required for reductional chromosome segregation at meiosis. Nature 1999; 400:461 - 464
- Heidinger-Pauli JM, Mert O, Davenport C, Guacci V, Koshland D. Systematic reduction of cohesin differentially affects chromosome segregation, condensation and DNA repair. Curr Biol 2010; 20:957 - 963
- McMurray MA, Gottschling DE. An age-induced switch to a hyper-recombinational state. Science 2003; 301:1908 - 1911