Abstract
Warburg effect and glutaminolysis are the two most noticeable metabolic features of tumor cells. A recent hypothesis is that tumor cells use glutamine as a preferred carbon source for energy and reducing power, which has been used to explain both Warburg effect and glutaminolysis. Here we provide evidence to demonstrate that supplying nitrogen, not the carbon skeleton, underlies the biological importance of glutaminolysis for proliferating cells. We show alternative nitrogen supplying mechanisms rescue Hep3B and HeLa cell proliferation in glutamine-free media. We conclude that cellular demand for nitrogen is a driving force of glutaminolysis for proliferating cells, and propose that glutamine addiction and Warburg effect are metabolic consequences of coordinated utilization of nitrogen and carbon sources for biosynthesis.
Introduction
The most remarkable metabolic features of tumor cells are the Warburg effect and glutamine (Gln) addiction.Citation1–Citation5 The Warburg effect was originally described as a seven- to nine-fold increase in glucose consumption by aerobic glycolysis to form lactate with a normal level of oxygen consumption.Citation1 This effect is different from the hypoxia-induced Pasteur effect, which reflects increased glycolysis to meet cells' energy needs when oxidative phosphorylation and the Krebs cycle are impaired.Citation6 However, the biological significance and underlying biochemical mechanism of the Warburg effect remain elusive. Increased glutaminolysis has been observed in some types of tumors.Citation7–Citation10 Glutaminolysis results in ammonia and Glutamate (Glu). Glu, in turn, can be further catabolyzed to α-ketoglutarate (α-KG) through either transamination or oxidative deamination. Notably, both catabolic pathways are reversible and involve the removal of nitrogen, being part of the mechanism that regulates nitrogen homeostasis. Since the carbon skeleton from glutaminolysis may eventually enter anabolic or anaplerotic processes, increased glutaminolysis in tumor cells has been explained by at least two models: (1) tumor cells are addicted to Gln as an alternative fuel, which is oxidized to CO2 for energy production;Citation11 and (2) α-KG derived from glutaminolysis enters the Krebs cycle to replenish metabolic intermediates removed for biosynthesis and, particularly, for the generation of NADPH and fatty acids. Collectively, both models emphasize the importance of the carbon skeleton from the glutaminolysis as a carbon source. Accordingly, excretion of the excessive carbons from glycolysis has been proposed as the cause of the Warburg effect.Citation12,Citation13
While the role of α-KG from glutaminolysis as an essential carbon source for tumor cells has been well accepted,Citation7,Citation11–Citation14 the importance of the Gln-Glu-α-KG axis in nitrogen anabolism during cell proliferation has not been fully appreciated. We examined the role of this axis in nitrogen metabolism in proliferating cells. We present data here to demonstrate that a central biological role of glutaminolysis in proliferating cells is to provide a source of nitrogen for the biosynthesis of nitrogenous biomolecules. We propose that maintaining the cytosolic pool of α-KG/Glu is essential for nitrogen dynamics during nitrogen anabolism. Since the inter-conversion between Glu and α-KG serves as a bridge connecting nitrogen and carbon metabolism, glutaminolysis and the Warburg effect are two integral parts of cellular machinery to balance the carbon and nitrogen anabolism.
Results
Gln is of universal necessity for cell proliferation.
It has been well established that the proliferation of HeLa and glioblastoma cells requires enhanced glutaminolysis.Citation7,Citation11,Citation15 We examined all cell lines that were in use in our laboratory, including 143B (osteosarcoma), 143B206, Hep3B (hepatoma) and MCF7 (breast carcinoma) and observed that all require Gln to proliferate (). Particularly, 143B206 is a ρ0 cell line derived from 143B.Citation16,Citation17 Lack of mitochondrial DNA leads to defective electron transfer chains in mitochondria, and so these cells rely exclusively on glycolysis for ATP production. 143B206 could maintain relatively slower but persistent proliferation in normal media, but stopped proliferating upon Gln depletion ( and B), suggesting glutaminolysis plays a critical role in cell proliferation that is independent of oxidative phosphorylation.
Gln cannot fully replace glucose as a carbon source to support prolonged cell proliferation.
The carbon skeleton from glutaminolysis is α-ketoglutarate (α-KG), which is glucogenic and plays a role in carbon metabolism. It is observed that most cells may continue to proliferate for a short duration after glucose withdrawal. However, since normal fetal bovine serum (FBS) contains glucose, whether Gln can serve as major carbon source to sufficiently support long term cell proliferation is unclear. We cultured various cell lines with glucose-free media supplemented with dialyzed FBS and observed that increasing amounts of Gln (up to 15 mM) failed to prevent eventual death of the cell lines tested, suggesting Gln is not sufficient to replace glucose as a major carbon source (). Therefore, both glucose and Gln are of fundamental necessity for cell proliferation, indicating an essential biological role of glutaminolysis that is distinct from supplying a carbon source to replace glucose.
Anaplerosis is not sufficient to support cell proliferation in Gln-free media.
The carbon skeleton of Gln is α-KG. Since α-KG cannot diffuse across the cell membrane, dimethyl-α-KG (DM-α-KG) has been used to increase the intracellular α-KG levels.Citation7 Upon entering cells, DM-α-KG is hydrolyzed to α-KG. It has been shown that 15 mM of DM-α-KG rescued glioblastoma proliferation in Gln-free media.Citation7 To test if DM-α-KG-provided rescue of cell proliferation in Gln-free media represents a general rule; we initially used 10 mM of DM-α-KG, but failed to rescue the proliferation of any cell lines we tested (not shown). A subsequent dose-curve analysis revealed that DM-α-KG indeed rescued Hep3B cell proliferation in Gln-free media, but this effect showed an intriguing biphasic modality with the optimal concentration at 2 mM (). We noted that HeLa was the first cell line reported to consume large amount of Gln, hence becoming the prototype of glutamine addiction.Citation11,Citation15 We observed that HeLa expressed high level of glutaminase () and confirmed that its proliferation depended on Gln, but DM-α-KG failed to rescue its proliferation in Gln-free media (). DM-α-KG also failed to rescue MCF7, 143B, 143B206 and 293 (not shown). Therefore, as the carbon-skeleton of glutaminolysis, α-KG rescues the proliferation in Gln-free media of specific cell types, including glioblastoma and Hep3B. Many other cell lines tested in our lab cannot be rescued by DM-α-KG in Gln-free media, regardless of their apparent dependence of glutaminolysis.
In addition to the energy source model, another proposed model is that glutaminolysis provides the carbon sources to replenish the Krebs cycle from which metabolites are removed for the production of NADPH and for other biosynthesis.Citation13 If this is true, alternative anaplerotic carbon sources shown in should be able to rescue Hep3B proliferation in Gln-free media. We supplemented Gln-free DMEM with various concentrations of dimethyl-succinate or diethyl-OAA, the membrane-permeable precursors of succinate and OAA, respectively.Citation18–Citation20 We observed that both failed to rescue the proliferation of Hep3B ( and F). We also tested diethyl-succinate, another membrane permeable precursor of succinate which also failed to rescue the cell proliferation (Sup. Fig. 1). We measured the effect of short-term Gln deprivation on succinate dehydrogenase activity, an indicator of the Krebs cycle activity.Citation21 After normalization with cell numbers independently determined by DNA contents, short-term Gln deprivation actually enhanced, not decreased the Krebs cycle activity (Sup. Fig. 2). These data indicate that the anaplerotic function of glutaminolysis is not the determining factor of cell proliferation.
Alternative nitrogen sources rescue Hep3B proliferation in Gln-free media.
In addition to α-KG, the other catabolic products of Gln are Glu, Asp, free ammonia and amino groups for other uses.Citation10,Citation22 Particularly, Gln depletion may directly lead to intracellular insufficiency of Gln, Glu and Asp. We next examined if nitrogen anabolism underlies the Gln-dependent proliferation. We used Hep3B, a tumor line originated from hepatocyte, which possesses adequate levels of enzymes to utilize Ala, Asp and even free ammonia as alternative nitrogen sources through pathways outlined in . If nitrogen deficiency is the cause of growth suppression in Gln-free media, supplementing the media with alternative nitrogen sources might be able to rescue the cell growth. After optimization, we observed that 4 mM of Ala rescued Hep3B proliferation in Gln-free medium (). Not surprisingly, pyruvate, the conjugate α-keto acid of Ala, which also is a product of glycolysis, showed little effect on cell proliferation (not shown). Moreover, Asn and Asp also supported Hep3B proliferation in Gln-free media (). Glu dehydrogenase catalyzes a reaction that is close to its equilibrium in cells.Citation23 While direct utilization of ammonia would be limited by membrane permeability and cytotoxicity, we predicted that a slight increase of ammonia might thermodynamically reverse the reaction towards the formation of Glu, hence at least facilitating the cell survival. We cultured Hep3B cells in Gln-free DMEM with 0.8 mM NH4+ + (optimized by dose curve, data not shown) for up to 6 days. Indeed, the presence of NH4+ + resulted in cell survival and slow proliferation (). Finally, the dose curve showed that 4 mM of Glu resulted in some rescuing effect that was far below optimal growth (). Increasing the concentrations (up to 10 mM) failed to further improve cell proliferation, showing a saturation pattern which is consistent with the notion that cellular uptake of Glu was limited by membrane transporter capacity.Citation24 We noted that Glu generated a plateau, but not biphasic modality as that observed with α-KG, consistent with the notion that cells uptake DM-α-KG and Glu with different mechanisms. Collectively, data presented above demonstrated that supply of alternative nitrogen sources is sufficient to support Hep3B cell survival and proliferation in Gln-free media.
Ala, Asp and α-KG-mediated rescue depends on transamination.
Both alternative nitrogen sources and DM-α-KG rescued Hep3B cell proliferation in Gln-free media. A common feature shared by glioblastoma and Hep3B is that both are derived from cells carrying out active nitrogen metabolism.Citation25 Since α-KG may serve as an acceptor of amino groups in transamination reactions, we hypothesized that α-KG rescued Hep3B proliferation by facilitating the utilization of the pre-existing nitrogen pool in the cells to form Glu. If this is true, the cell-type-specific rescuing effect of α-KG might depend on the collective activities of transaminases to produce Glu, and the rescuing effects of Ala and Asp will largely depend on the cytosolic ALT1 and AST1 activity, respectively. Supporting this hypothesis, Ala and α-KG showed a synergetic rescuing effect on Hep3B proliferation (). Since complete inhibition of aminotransferase activity leads to cell death, we used low doses of transaminase inhibitor aminooxyacetic acid (AOAA) to partially repress transamination. While AOAA at these concentrations showed little effect on cell proliferation in complete media, it significantly impaired DM-α-KG-mediated rescue (). Additional assays indicated that except for Hep3B, none of the HeLa, MCF7 and 143B lines were rescued by Ala, Asp or Asn ( and Sup. Fig. 3). Quantitative RT-PCR analyses confirmed that Hep3B expressed ALT1/2 and AST1/2 mRNAs, with their relative abundance shown in Supplemental . Specifically, ALT1 and AST1 are reported to catalyze the Ala-Glu and Asp-Glu transaminations respectively in cytosol.Citation26–Citation28 The failure of rescue by Ala or Asp would predict relatively low levels of ALT1 and AST1 in a cell. Indeed, the cell lines that could not be rescued by Ala expressed barely detectable ALT1 and cells that showed less response to Asp had significantly lower AST1, as determined by Real-Time RT-PCR analysis (). We cloned and overexpressed ALT1 in HeLa cells () and these cells gained the ability to proliferate in Gln-free media supplemented with Ala and α-KG (). These data indicate that aminotransferase activity facilitates α-KG-rescued cell proliferation.
Gln depletion triggers amino acid deprivation stress and adaptive response.
To determine if Gln depletion caused amino acid deprivation stress in cultured cells, we examined the level of phosphorylated eIF2α (Ser51)Citation29,Citation30 and obsrved that Gln withdrawal induced eIF2α (Ser51) phosphorylation in all cell lines tested (). Next, we examined the transcriptional response of genes encoding key enzymes of nitrogen metabolism by qRT-PCR. Overnight Gln depletion enhanced the expression of Glu dehydrogenase and glutaminase 1 and 2, three enzymes directly involved in Glu homeostasis (). These data indicate that Gln depletion causes amino acid deprivation stress and transcriptionally modulates Glu homeostasis in cultured cells.
Ammonia supports long-term proliferation of Hep3B.
We cultured Hep3B in Gln-free media with dialyzed FBS and 0.8 mM ammonia. Cells cultured in this media have survived and continued to proliferate for more than five months. Cells in the same medium but without ammonia died out completely after three weeks. For easy description, we named the cells grown on ammonia MM01. When compared with Hep3B, MM01 showed no obvious morphological difference under light microscopy (). Despite the apparently similar morphology, MM01 cells showed a lower proliferation rate than Hep3B (). While started with approximately the same number of cells in each plate (about 5 × 104), MM01 had a visibly lower cell density after 3 days (). Withdrawal of ammonia stopped MM01 proliferation and induced cell death (), indicating ammonia was a bona fide nitrogen source for MM01. Unlike Hep3B exposed to Gln deprivation, MM01 cells have Krebs cycle activity similar to Hep3B in normal media (). Finally, qRT-PCR analysis revealed that MM01 had elevated expression of genes involved in Glu homeostasis (). The elevated expression of ALT2 and Gln synthetase indicates MM01 uses adaptive nitrogen anabolic pathways to synthesize Ala and Gln (). Therefore, while ammonia is not an optimal nitrogen source, it is sufficient to support long-term proliferation of Hep3B with adaptive nitrogen anabolism.
Effects of nitrogen source restriction on carbon metabolism and nucleic acid biosynthesis.
Proliferating cells have an active nucleic acid synthesis process and require a large quantity of nucleotides supply. De novo biosynthesis of nitrogenous bases in the cell directly uses Asp, Glu, Gly and Gln as substrates at various steps. To clarify whether impaired nucleotide synthesis by lack of Gln is the major cellular process that limits cell proliferation, we tested the rescuing effects of precursors or intermediate metabolites of nucleotide biosynthesis on cell proliferation (Sup. Fig. 5). Data shown indicated that alternative biosynthesis pathways of purine and pyrimidine failed to significantly rescue cell proliferation in Gln-free medium, suggesting nitrogen anabolism is essential for multiple metabolic processes during cell proliferation.
To explore the effects of nitrogen source restriction on carbon metabolism, we analyzed the mRNA expression of key enzymes of several major carbon metabolic pathways (). Based on the qRT-PCR data, all enzymes tested were transcriptionally downregulated. Specifically, at acute Gln deprivation, both HK2 and PFK1 were repressed dramatically, while G6PD was less affected, suggesting that acute nitrogen deprivation mainly affects glycolysis. MM01 cell line growing in Gln-free media with ammonia represents an adaptation to long-term nitrogen-insufficiency. Under this culture condition, the expression level of PFK1 was partially recovered, but G6PD, citrate synthetase and FAS were further downregulated. These data indicate that nitrogen deprivation or insufficiency transcriptionally slows down glucose flux to glycolysis and represses other carbon metabolic pathways, but are consistent with a recent report that glutaminolysis influences the glucose uptake.Citation31
Discussion
Metabolism is essential for every cellular process and so the basic principles and pathways of metabolism have been extensively studied under physiological conditions. In recent years, it has become clearer and clearer that a thorough understanding of the metabolic changes under various pathological conditions holds the key to a better management and prevention of diseases.Citation13,Citation22,Citation32 Two of the most noticeable metabolic alterations in proliferating cells are the Warburg effect and glutaminolysis. Both are driven by various oncogenic pathways;Citation22,Citation32 however, the proliferative advantages of the Warburg effect and glutaminolysis remain unclear. Based on the fact that proliferating cells require active biosynthesis, which requires a carbon source for both the synthesis of the carbon skeleton and production of energy, proposed advantages of glutaminolysis include providing an alternative energy source for tumor cells.Citation33 Since biosynthesis also requires reducing power in the form of NADPH in large quantity, a recent hypothesis is that in proliferating cells, the dramatically increased need of NADPH cannot be met by the pentose pathway.Citation13 This metabolic imbalance leads to exporting malate and citrate from mitochondria to the cytosol for NADPH production. In addition, active synthesis of other molecules, such as fatty acids, also consumes metabolites of the Krebs cycle. Accordingly, the importance of glutaminolysis in proliferating is proposed to replenish the Krebs cycle with α-KG, and the Warburg effect facilitates the disposal of excessive carbons from glycolysis.Citation13
The Gln-Glu-α-KG axis plays a central role in both nitrogen and carbon metabolism.Citation22,Citation32,Citation34 Gln is a major transport form of nitrogen in vivo, being the most abundant amino acid in circulation.Citation35 Indeed DM-α-KG rescues Hep3B proliferation, and α-KG from glutaminolysis can be utilized by the Krebs cycle. However, it is also proposed that reduced, not enhanced, Krebs cycle activity and oxidative phosphorylation provides proliferative advantage for tumor growth in vivo.Citation36–Citation38 While providing an energy source and anaplerotic substrate for tumor cells may have a proliferative advantage for tumor cells, it might not be the most essential role of glutaminolysis for cell proliferation. This argument is based on the following facts: (1) Cell permeable precursors of other anaplerotic-intermediate metabolites, including OAA and succinate, failed to rescue the repression; (2) That ρ0 cells are also addicted to glutaminolysis suggests Gln has a role more critical than energy generation through oxidative phosphorylation; (3) The mutations of SDH and fumarate hydratase in some cancers indicates that a complete Krebs cycle is not absolutely required for cell proliferation;Citation39–Citation42 and finally, (4) The need for NADPH for biosynthesis could be met by the pentose pathway, and there is no real necessity to use Gln for NADPH. It is proposed that oxidation of one mole of glucose via the pentose pathway only generates two moles of NADPH, which may cause metabolic imbalance during proliferation. We believe that cells can always oxidize more glucose, which is normally abundant both in vivo and in cultured cells, to generate more NADPH. Resulting ribose phosphate that exceeds biosynthetic needs can be recycled to hexose through non-oxidative pathways so the pentose pathway can continue producing NADPH as much as needed. For fatty acid synthesis, exporting of citrate out of mitochondria provides an additional source of NADPH. The mitochondrial synthesis of citrate is critical for cell proliferation, but it can be completed by using Glc as the sole carbon source where the pyruvate-to-OAA pathway provides the anapleurotic function.
We observed that Gln depletion suppressed the proliferation of all cell lines tested, consistent with results reported by other researchers.Citation7,Citation11,Citation43 However, our data indicate that lack of Glu as a nitrogen supply may be a more important cause of growth arrest. Data supporting this view include: (1) Gln withdrawal causes amino acid deprivation response and transcriptionally regulates genes of nitrogen metabolic pathways; (2) Cell proliferation in Gln-free media can be restored by alternative nitrogen sources such as Ala, Asp or Asn; (3) While DM-α-KG partly rescues this repression, its rescuing effect requires transaminase activity; (4) The rescuing effect of DM-α-KG can be significantly improved by simultaneous addition of Ala, Asp or Asn; and (5) Ammonia is sufficient to support proliferation of Hep3B which expresses adequate Glu dehydrogenase. Eventual confirmation of this model requires a precise determination of cytosolic Glu levels.
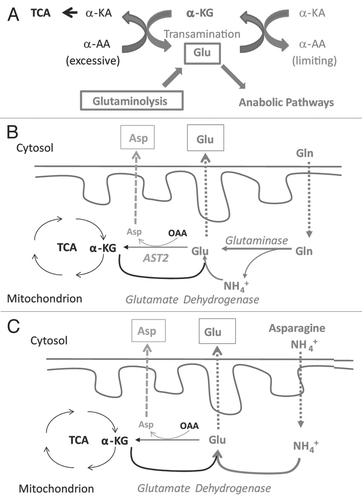
Based on our data presented here and a careful reviewing of data published by others in recent years and decades ago, we propose an alternative model to explain the biological importance of glutaminolysis in supporting the systematic biosynthesis required for cell proliferation and its relevance to the Warburg effect (). This model is based on the fact that biosynthesis in proliferating cells demands active metabolism of both carbon and nitrogen. Glucose provides a good carbon source for glycolysis and pentose shunt, whereas amino acids, particularly Gln, are the major nitrogen source imported into the cells. In the cytosol, inter-conversion between Glu, and α-KG plays the central role in maintaining a dynamically balanced pool of amino acids to support nitrogen-dependent synthesis (). Amino groups of excess amino acids are collected by α-KG to form Glu and non-essential amino acids in demand will be synthesized by transamination between their α-keto acids (α-KA) and Glu. Maintaining a dynamic balance of α-KG and Glu pool in the cytosol is crucial for this function, which is complicated by the fact that cytosolic Glu, along with its carbon skeleton α-KG, is consumed by several major pathways in proliferating cells: (1) being directly consumed in protein and nucleotide synthesis; (2) forming Gln, which is used in the synthesis of protein and nucleotides; (3) being used in biosynthesis of ornithine, which is used to synthesize Arg and polyamines required for DNA replication and chromatin structure; and (4) biosynthesis of glutathione, a key abundant molecule protecting cells from oxidative damage. Therefore, in proliferating cells, there is a considerably large demand for Glu. The dilemma is that for most cell types, direct uptake of Glu is limited by the scarce expression of Glu transporter. Some cell lines even express a special type transporters to actively release Glu to the environment.Citation24,Citation44,Citation45 This dilemma is solved by uptaking Gln, which is very abundant in normal plasma and subsequently converting it to Glu by glutaminolysis ( and B). Mitochondrial Glu has three fates: (1) being exported to cytosol, contributing to the cytosol Glu pool; (2) being transaminated with OAA, forming α-KG and Asp; and (3) being oxidatively deaminated, forming α-KG, NAD(P)H and NH4+. The last fate mainly serves as a part of the urea cycle in hepatocytes. Accordingly, compared with Hep3B, HeLa cells have much higher level of glutaminase, but relatively low levels of Glu dehydrogenase ( and C). In addition, considering that proliferating cells demand increased amounts of Glu and Asp, oxidative deamination is unlikely a major fate of Gln in most non-hepatic tumor cells. This is consistent with the fact that Myc signaling upregulates the expression of Gln transporters and glutaminase, but not Glu dehydrogenase.Citation43,Citation46
The proposed Gln-Glu/Asp exchange may not be the major fate of α-KG from glutaminolysis, but could be the essential mechanism for the proliferation of most tumor cells (). At organismal levels, Gln, Glu and Asp are considered as non-essential amino acids. At the cellular level, when Gln is absent, it needs to be synthesized from Glu and Glu needs to be synthesized from α-KG via two pathways: (1) The reverse reaction of oxidative deamination, catalyzed by Glu dehydrogenase with NAD(P)H as reducing power and ammonia as the nitrogen source; and (2) transam-ination reactions with other amino acids. Importantly, active synthesis of Glu via these two pathways is limited to special cell types that express the respective enzymes to catalyze these reactions. Hepatocytes and Hep3B cells, for example, have ample levels of cytosolic ALT1 and AST1, hence being rescued by Ala and Asp/Asn ( and C). Therefore, at cellular level, Gln is an essential amino acid for cells that are not able to either synthesize or uptake a sufficient amount Glu from media in cell culture or from plasma in vivo. Clinically, the L-asparaginase treatment of leukemia, a therapy based on depleting L-Asn from tumor cells, is frequently complicated with a depletion of Gln, but not other amino acids, from the patient plasma,Citation29 which is consistent with our model that Gln is the major nitrogen source for the synthesis of non-essential amino acids in most tumor cells.
Although the direct anabolism of nitrogen from ammonia by specific cell types also has been demonstrated by isotope tracing studiesCitation47 and ammonia can support long term cell proliferation, the MM01 proliferation is relatively slow. This indicates that direct utilization of ammonia by reductive amination of α-KG is not an effective way to synthesize Glu (). For most cell types that cannot synthesize sufficient amount of Glu from α-KG, glutaminolysis becomes critical to maintain the Glu pool. Deprivation of Gln will lead to the lack of Gln, Glu and α-KG in cytosol. Both alternative pathways to generate Glu require α-KG as a substrate, providing an alternative explanation for DM-α-KG-mediated rescue of Hep3B and glioblastoma cell proliferation in Gln-free media. We also observed that excessive α-KG repressed Hep3B proliferation (). While the biochemical mechanism underlying this repression remains unclear, it is possible that excessive α-KG disturbs the dynamics of amino acids pool by competitively inhibiting transamination between Glu and other α-KAs.
Non-essential amino acids Asp and Ala are synthesized from OAA and pyruvate, respectively, by transamination with Glu. Particularly, Ala is the second most abundant amino acid, accounting for about 8% of the primary structure based on an analysis of protein sequences.Citation48 Asp is directly consumed in biosynthesis of nucleotides. Biosynthesis of Ala and Asp may have a significant influence on the intracellular Glu pool. Increased supply of Ala and Asp may spare the Glu pool, hence partially alleviating the needs of Glu. This may partly contribute to their effectiveness in rescuing cell proliferation in Gln-free media.
In summary, cell proliferation dictates active and systematic biosynthesis of lipids, proteins and nucleotides. Carbon, nitrogen and oxygen sources are equally important for proliferative biosynthesis and the essential role of glutaminolysis is to maintain the intracellular levels of Glu and to serve as a nitrogen source. While the HIF pathway has been identified as a major regulator of glucose and oxygen metabolism,Citation49,Citation50 the Myc pathway has been identified to stimulate glutaminolysis.Citation7,Citation43 The functional interaction between HIF and Myc pathways may coordinately regulate the metabolism of carbon, oxygen and nitrogen and balance the proliferative needs of energy, reducing power and structural elements.Citation34 How cells sense the intracellular levels of nitrogen source requires further investigation. A thorough understanding of the regulation of glutaminolysis and particularly its coordinated control with carbon and oxygen utilization may bring significant insight into the biochemical basis of cell proliferation.
Methods
Cell culture reagents, cell lines and regular maintenance.
Cell culture media and ingredients were purchased from Mediatech. Amino acids, DM-α-KG, DM-succinate, diethyl-OAA, uridine, orotate and hypoxanthine were from Sigma-Aldrich. Hep3B, HeLa and MCF-7 cell lines were from ATCC. 143B and 143B-206 cell lines were kind gifts from Dr. Michael King (Thomas Jefferson University).
Cell growth curve and mitochondrial activity assays.
Dialyzed (10 kD cut) FBS was used in all growth curve assays (Atlantic Biologicals). Cell proliferation was determined by using CyQUANT® NF-Cell Proliferation Kit (Invitrogen). Succinate dehydrogenase activity was determined by using methylthiazolyldiphenyl-tetrazolium bromide.
qRT-PCR.
Total RNA was extracted with Qiagen RNeasy kit. cDNA was synthesized by Invitrogen superscript II reverse transcriptase with random hexamers as primers. qRT-PCR was performed with Taqman gene expression master mix and StepOnePlus Real-Time PCR System (Applied Biosystems). Data were quantitatively analyzed by StepOne software with comparative CT method. β-actin was set as endogenous control.
Western blotting.
All processes were previously described.Citation51,Citation52 Anti-glutaminase antibody was from ABChem. Antiphopshorylated (Ser51) and total eIF-2α antibodies were from Cell Signaling Technology.
Establishment of cell line growing on ammonia (MM01).
Hep3B cells were seeded in Gln-free DMEM with 10% dialysed FBS (Atlantic Biologicals, dialysed 10 kD) and 0.8 mM ammonia. The medium was changed every 2 days and cells were passed when reaching 90% confluence. The cells cultured in Gln-free media with ammonia were maintained as such thereafter.
Microscopy.
Images of living cells were photographed under inverted Olympus IX81 Nomarski microscope by using 100× or 200× magnification.
Statistical analysis.
Student's t-test was used. p < 0.01 was set as significant (indicated by ** in figures).
Authors Contribution
N.S. proposed and modified hypothesis, designed experiments, analyzed data, wrote manuscript; M.M. designed and set-up experiments, analyzed and organized data, wrote manuscript; S.C. carried out western blotting, growth curve and qRT-PCR analysis; T.L. studied the cell morphology; D.L. cloned and expressed ALT1/2, AST1/2 genes in HeLa cells and selected for stable clones.
Abbreviations
| AA | = | amino acids |
| Ala | = | alanine |
| ALT | = | alanine aminotransferase |
| AOAA | = | aminooxyacetic acid |
| Arg | = | arginine |
| Asn | = | asparagine |
| Asp | = | aspartate |
| AST | = | aspartate aminotransferase |
| DM-α-KG | = | dimethy-α-ketoglutarate |
| GLUD | = | glutamate dehydrogenase |
| Glc | = | glucose |
| Gln | = | glutamine |
| Glu | = | glutamate |
| Gly | = | glycine |
| OAA | = | oxaloacetate |
| qRT-PCR | = | quantitative real-time polymerase chain reaction |
| α-KA | = | α-keto acid |
| α-KG | = | α-ketoglutarate |
Figures and Tables
Figure 1 All cell lines tested require Gln for proliferation. Cells were seeded in Gln-free DMEM supplemented with 0 or 4 mM Gln. Cell numbers were analyzed every 2 days and mean values were shown. Representative growth curves of 143B (A), 143B206 (B), Hep3B (C) and MCF7 (D) cells are shown. Error bars indicate SE of ≥4 replicates.
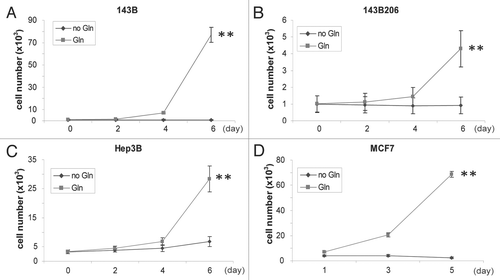
Figure 2 All cell lines tested require glucose for proliferation. Cells were seeded in Gln-free DMEM supplemented with 0 or 4.5 g/L Glc and 5 or 10 mM Gln, respectively. Cell numbers were analyzed every 2 days and mean values were shown. Representative growth curves of Hep3B (A), Hela (B), MCF7 (C) and 143B (D) cells are shown. Error bars indicate SE of ≥4 replicates.
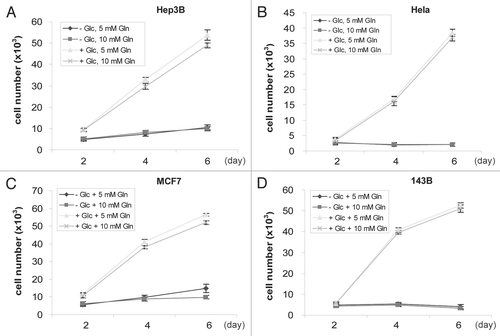
Figure 3 Rescuing effect of DM-α-KG is not based on anaplerosis. Increasing concentrations of DM-α-KG were used in cell culture. Mean value of cell number was shown. Error bars indicate SE of ≥4 replicates. (A) Biphasic effect of DM-α-KG on proliferation of Hep3B cells. (B) Protein levels of glutaminase in HeLa and Hep3B cells. 80 µg of total proteins were loaded onto a SDS page. Glutaminase levels were determined by western blotting. α-tubulin was used as a loading control. (C) α-KG failed to rescue HeLa cell proliferation in Glu-free media. (D) Simplified drawing showing the major anaplerotic metabolites of TCA cycle. In addition to α-KG, succinate and OAA have anaplerotic roles. (E and F) Growth curve of Hep3B cells in Gln-free DMEM supplemented with either 1–8 mM of diethyl-OAA or dimethyl-succinate. DMEM and complete DMEM (+Gln) were respectively set as controls.
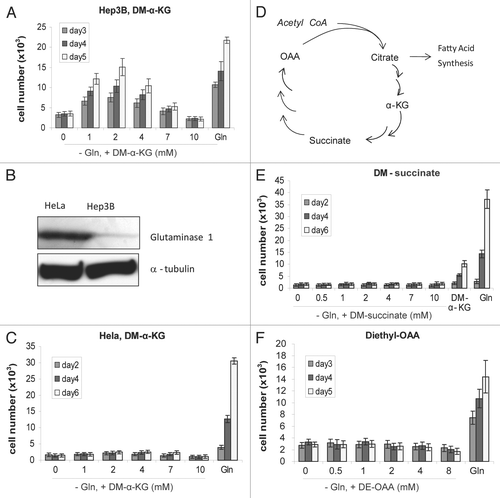
Figure 4 Alternative nitrogen sources rescue Hep3B. (A) Nitrogen metabolic pathways relevant to this study. Glutaminolysis and the metabolic pathways to utilize Ala, Asp, Asn and ammonia as nitrogen sources were shown. (B–D) Growth curve of Hep3B cells in Gln-free DMEM supplemented with 4 mM Asns or Asp (B), with 4 mM Ala (C), with 0.8 mM ammonia or 4 mM Glu (D). For (B–D) cells seeded in Gln-free DMEM were used as control. Cell number was analyzed every 2 or 3 days and mean values were shown. (E) Dose curve of Hep3B cells in various concentrations of Glu.
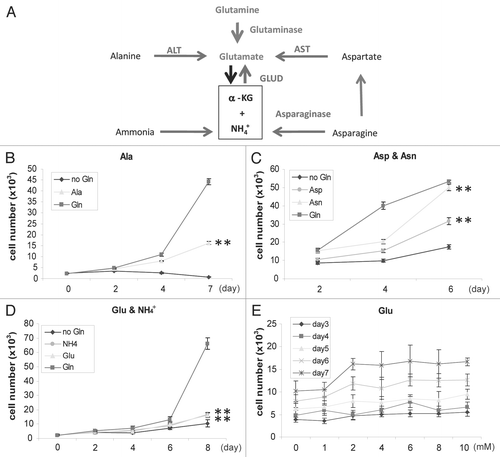
Figure 5 Rescuing effects of α-KG, Ala and Asp depend on transamination. (A) Synergetic rescuing effect of Ala and α-KG. Growth curve of Hep3B cells in Gln-free DMEM supplemented with DM-α-KG (2 mM) or a combination of DM-α-KG (2 mM) and Ala (4 mM). Mean value of cell number was shown with plotted lines. Error bars indicate SE of ≥4 replicates. (B) Presence of transaminase inhibitor impairs the rescuing effects of α-KG. AOAA was used at 0.1 and 0.2 mM. Data represent percentage of cell numbers compared with those in normal media at day 4. Cell numbers with normal media were set as 100%. (C) HeLa cells cannot be rescued by either α-KG or alternative nitrogen sources. (D) q-RT-PCR analysis showing ALT1 and AST1 levels are associated with the rescuing effect. (E) Exogenous expression of ALT1 in HeLa cells. (F) Expression of ALT1 in combination with Ala and α-KG rescues HeLa proliferation. HeLa cells transfected with vector or ALT1-expressing plasmids were grown in Gln-free media for 7 days. Representative images were taken (200×).
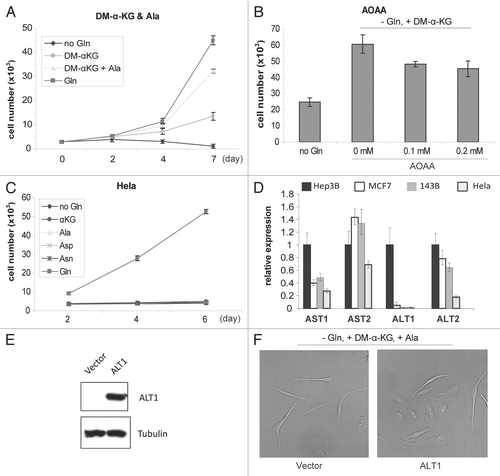
Figure 6 Gln depletion triggers amino acid deprivation response and adaptive expression of genes important for Glu metabolism. (A) Immunoblotting analysis of phosphorylated-(Ser51) eIF2α, a biomarker of amino acid deprivation stress response. Total eIF2α was detected as control. (B) Gln depletion upregulates genes involved in nitrogen metabolism. Cells were cultured in media with or without Gln for 48 h, and total RNA samples were prepared. The mRNA levels of genes encoding major nitrogen metabolic enzymes were analyzed by real-time RT-PCR.
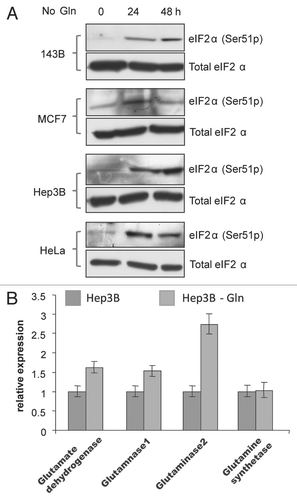
Figure 7 Establishment of cell lines growing in ammonia-containing media. (A) Morphology of MM01 and Hep3B (200×). (B) Ammonia is sufficient to support long-term proliferation of Hep3B in Gln-free media. Hep3B cells were seeded in complete media (three top parts) as control. MM01 cells were seeded in Gln-free media with ammonia (three middle parts). Withdrawal of ammonia suppressed proliferation (three bottom parts). All micrographs were taken at 100× magnification. (C) Mitochondrial activity of Hep3B and MM01 as determined by MTT assays. (D) Adaptive expression of genes encoding major enzymes involved in Glu homeostasis. (E) Change of expression of ALT1/2 and Gln synthetase were determined by real-time RT-PCR. Data indicate that MM01 growing on ammonia-expressing genes facilitate nitrogen flux to the synthesis of Ala (ALT2) and Gln (glutamine synthetase).
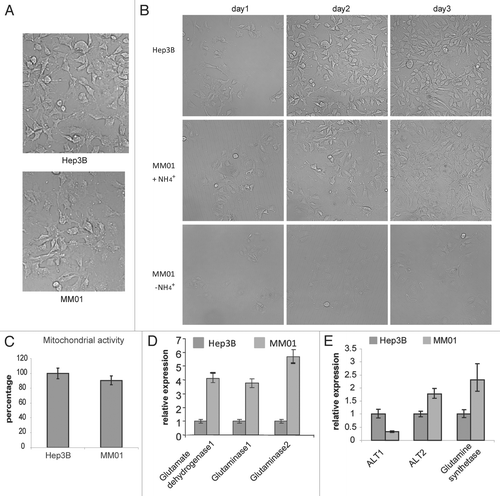
Figure 8 Nitrogen restriction represses the utilization of glucose. Relative mRNA levels of genes involved in glucose utilization were determined by qRT-PCR. Hep3B cells grown in normal DMEM medium were set as reference. (A) Relative gene expression levels in Hep3B cells treated by Gln-free DMEM for 12 hours. (B) Relative gene expression level in MM01 cells grown in Gln-free DMEM supplemented with 0.4 mM (NH4)2SO4. HK2, hexokinase 2; PFK1, phosphofructokinase 1; CS, citrate synthase; G6PD, glucose-6-phosphate dehydrogenase; FASN, fatty acid synthase; ACACA, acetyl-CoA carboxylase.
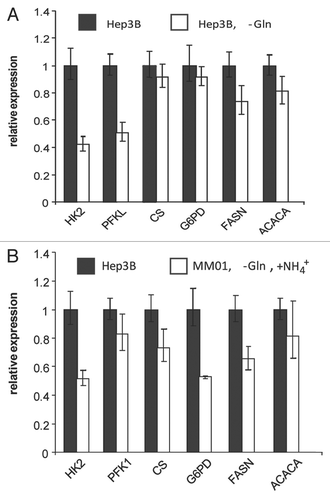
Figure 9 Proposed role of glutaminolysis in nitrogen anabolism in proliferating cells. (A) Central roles of interconversion between α-KG and Glu in nitrogen metabolism. The interconversion between α-KG and Glu in cytoplasmic provides a mechanism to collect amino groups from excessive amino acids and redistribute them to produce amino acids on demand. An adequate pool of cytoplasmic α-KG and Glu is essential for this function. In proliferating cells, Glu is actively consumed for biosynthesis. Because the uptake of Glu is limited by the capacity of cell surface transporters, the loss of cytoplamic gluatamate is mainly replenished by glutaminolysis. Excessive amino acids may replenish the Glu pool, contingent on the transaminase acitivity. Excessive α-KG inhibits transamination from Glu to other α-KAs, impairing the biosynthesis of amino acids in demand. (B) Glutaminolysis and Gln-Glu/Asp exchange model. When Gln is available, Gln enters mitochondria and is hydrolyzed to Glu. In addition to being consumed by the Krebs cycle, mitochondrial Glu can exported outside to maintain the homeostasis of the cytoplasmic Glu-α-KG pool or used to generate Asp for export. (C) Recycling of ammonia for nitrogen anabolism by specific cell types. When Gln is scarce, some types of cells may use ammonia (in media or from catabolism of amino acids such as Asn) to synthesize Glu. This pathway depends on the GLUD activity. α-KA, α-keto acids; α-AA, α-amino acids.
Additional material
Download Zip (978.5 KB)Acknowledgements
We thank Dr. M. King for the 143B and 143B206 (ρ0) cell lines. We thank Dr. J. Bentz and Mr. V. Duggirala for discussion and proofreading of the manuscript. This work is supported in part by grants K01-CA098809 and R01-CA129494 (to N.S.) from NCI, National Institutes of Health (NIH) and start-up funds from Drexel University.
References
- Warburg O. On the origin of cancer cells. Science 1956; 123:309 - 314
- Klimberg VS, McClellan JL, Organ CH Jr. Honorary Lectureship. Glutamine, cancer and its therapy. Am J Surg 1996; 172:418 - 424
- Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, et al. The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 2009; 8:3984 - 4001
- Medina MA. Glutamine and cancer. J Nutr 2001; 131:2539 - 2542
- Souba WW. Glutamine and cancer. Ann Surg 1993; 218:715 - 728
- Ghosh AK, Sloviter HA. Glycolysis and pasteur effect in rat reticulocytes. J Biol Chem 1973; 248:3035 - 3040
- Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA 2008; 105:18782 - 18787
- Medina MA, Nunez de Castro I. Glutaminolysis and glycolysis interactions in proliferant cells. Int J Biochem 1990; 22:681 - 683
- Segura JA, Medina MA, Alonso FJ, Sanchez-Jimenez F, Nunez de Castro I. Glycolysis and glutaminolysis in perifused Ehrlich ascites tumour cells. Cell Biochem Funct 1989; 7:7 - 10
- Aledo JC. Glutamine breakdown in rapidly dividing cells: waste or investment?. Bioessays 2004; 26:778 - 785
- Reitzer LJ, Wice BM, Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem 1979; 254:2669 - 2676
- Hitosugi T, Kang S, Vander Heiden MG, Chung TW, Elf S, Lythgoe K, et al. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal 2009; 2:73
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009; 324:1029 - 1033
- DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, et al. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA 2007; 104:19345 - 19350
- Blaker GJ, Birch JR, Pirt SJ. The glucose, insulin and glutamine requirements of suspension cultures of HeLa cells in a defined culture medium. J Cell Sci 1971; 9:529 - 537
- King MP, Attardi G. Human cells lacking mtDNA: Repopulation with exogenous mitochondria by complementation. Science 1989; 246:500 - 503
- Procaccio V, Mousson B, Beugnot R, Duborjal H, Feillet F, Putet G, et al. Nuclear DNA origin of mitochondrial complex I deficiency in fatal infantile lactic acidosis evidenced by transnuclear complementation of cultured fibroblasts. J Clin Invest 1999; 104:83 - 92
- Malaisse WJ, Ladriere L, Jijakli H, Laatikainen R, Niemitz M, Verbruggen I, et al. Metabolism of the dimethyl ester of [2,3-(13)C]succinic acid in rat hepatocytes. Mol Cell Biochem 1998; 189:137 - 144
- Koivunen P, Hirsila M, Gunzler V, Kivirikko KI, Myllyharju J. Catalytic properties of the asparaginyl hydroxylase (FIH) in the oxygen sensing pathway are distinct from those of its prolyl 4-hydroxylases. J Biol Chem 2004; 279:9899 - 9904
- Zlotnik A, Gruenbaum SE, Artru AA, Rozet I, Dubilet M, Tkachov S, et al. The neuroprotective effects of oxaloacetate in closed head injury in rats is mediated by its blood glutamate scavenging activity: evidence from the use of maleate. J Neurosurg Anesthesiol 2009; 21:235 - 241
- Soderberg KL, Ditta GS, Scheffler IE. Mammalian cells with defective mitochondrial functions: A Chinese hamster mutant cell line lacking succinate dehydrogenase activity. Cell 1977; 10:697 - 702
- Dang CV. Rethinking the Warburg effect with Myc micromanaging glutamine metabolism. Cancer Res 2010; 70:859 - 862
- Silverstein E, Sulebele G. Equilibrium kinetic study of the catalytic mechanism of oxidative deamination of alanine by bovine liver glutamate dehydrogenase. Biochemistry 1974; 13:1815 - 1818
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 1996; 16:675 - 686
- Hertz L, Dringen R, Schousboe A, Robinson SR. Astrocytes: glutamate producers for neurons. J Neurosci Res 1999; 57:417 - 428
- Safer B, Williamson JR. Mitochondrial-cytosolic interactions in perfused rat heart. Role of coupled transamination in repletion of citric acid cycle intermediates. J Biol Chem 1973; 248:2570 - 2579
- Yang R-Z, Park S, Reagan WJ, Goldstein R, Zhong S, Lawton M, et al. Alanine aminotransferase isoenzymes: molecular cloning and quantitative analysis of tissue expression in rats and serum elevation in liver toxicity. Hepatology 2009; 49:598 - 607
- Yang RZ, Blaileanu G, Hansen BC, Shuldiner AR, Gong DW. cDNA cloning, genomic structure, chromosomal mapping and functional expression of a novel human alanine aminotransferase. Genomics 2002; 79:445 - 450
- Bunpo P, Dudley A, Cundiff JK, Cavener DR, Wek RC, Anthony TG. GCN2 protein kinase is required to activate amino acid deprivation responses in mice treated with the anti-cancer agent L-asparaginase. J Biol Chem 2009; 284:32742 - 32749
- Zhang P, McGrath BC, Reinert J, Olsen DS, Lei L, Gill S, et al. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol Cell Biol 2002; 22:6681 - 6688
- Kaadige MR, Looper RE, Kamalanaadhan S, Ayer DE. Glutamine-dependent anapleurosis dictates glucose uptake and cell growth by regulating MondoA transcriptional activity. Proc Natl Acad Sci USA 2009; 106:14878 - 14883
- Dang CV. PKM2 tyrosine phosphorylation and glutamine metabolism signal a different view of the Warburg effect. Sci Signal 2009; 2:75
- Yuneva M. Finding an “Achilles' heel” of cancer: The role of glucose and glutamine metabolism in the survival of transformed cells. Cell Cycle 2008; 7:2083 - 2089
- Dang CV. The interplay between MYC and HIF in the Warburg effect. Ernst Schering Found Symp Proc 2007; 35 - 53
- Yang H, Roth CM, Ierapetritou MG. A rational design approach for amino acid supplementation in hepatocyte culture. Biotechnol Bioeng 2009; 103:1176 - 1191
- Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab 2006; 3:187 - 197
- Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer 2008; 8:705 - 713
- Chen Y, Cairns R, Papandreou I, Koong A, Denko NC. Oxygen consumption can regulate the growth of tumors, a new perspective on the Warburg effect. PLoS One 2009; 4:e7033
- Pollard PJ, El-Bahrawy M, Poulsom R, Elia G, Killick P, Kelly G, et al. Expression of HIF-1alpha, HIF-2alpha (EPAS1) and their target genes in paraganglioma and pheochromocytoma with VHL and SDH mutations. J Clin Endocrinol Metab 2006; 91:4593 - 4598
- Pollard PJ, Briere JJ, Alam NA, Barwell J, Barclay E, Wortham NC, et al. Accumulation of Krebs cycle intermediates and overexpression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet 2005; 14:2231 - 2239
- Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell 2005; 7:77 - 85
- Isaacs JS, Jung YJ, Mole DR, Lee S, Torres-Cabala C, Chung YL, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: Novel role of fumarate in regulation of HIF stability. Cancer Cell 2005; 8:143 - 153
- Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 2009; 458:762 - 765
- Haussinger D, Gerok W. Hepatocyte heterogeneity in glutamate uptake by isolated perfused rat liver. Eur J Biochem 1983; 136:421 - 425
- Lo M, Wang YZ, Gout PW. The x(c)-cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J Cell Physiol 2008; 215:593 - 602
- Dang CV. MYC, microRNAs and glutamine addiction in cancers. Cell Cycle 2009; 8:3243 - 3245
- Sheng YB, Badger TM, Asplund JM, Wixom RL. Incorporation of 15NH4Cl into histidine in adult man. J Nutr 1977; 107:621 - 630
- Doolittle RF. Protein sequence comparisons: searching databases and aligning sequences. Curr Opin Biotechnol 1994; 5:24 - 28
- Liang D, Kong X, Sang N. Effects of histone deacetylase inhibitors on HIF-1. Cell Cycle 2006; 5:2430 - 2435
- Semenza GL. HIF-1 mediates the Warburg effect in clear cell renal carcinoma. J Bioenerg Biomembr 2007; 39:231 - 234
- Fath DM, Kong X, Liang D, Lin Z, Chou A, Jiang Y, et al. Histone deacetylase inhibitors repress the transactivation potential of hypoxia-inducible factors independently of direct acetylation of HIFalpha. J Biol Chem 2006; 281:13612 - 13619
- Stiehl DP, Fath DM, Liang D, Jiang Y, Sang N. Histone deacetylase inhibitors synergize p300 autoacetylation that regulates its transactivation activity and complex formation. Cancer Res 2007; 67:2256 - 2264