Abstract
Cyclin E is a key component of the cell cycle regulatory machinery, contributing to the activation of Cdk2 and the control of cell cycle progression at several stages. Cyclin E expression is tightly regulated, by periodic transcription and ubiquitin-mediated degradation. Overexpression of cyclin E has been associated with tumor development and poor prognosis in several tumor types, including germ cell tumors, and both cyclin E and its partner Cdk2 are required for normal spermatogenesis. Here we have generated and characterized transgenic mice overexpressing a cyclin E mutant protein, resistant to ubiquitin-mediated proteolysis, in testicular germ cells, under the control of the human EF-1 alpha promoter. The transgenic mice develop normally and live a normal life span, with no signs of testicular tumor development. The transgenic mice display however reduced fertility and testicular atrophy, due to reduced spermatogonial proliferation as a consequence of deregulated cyclin E levels. Overall our results show that deregulation of cyclin E expression contribute to infertility, due to inability of the spermatogonial cells to start the mitotic cycles prior to entering meiosis.
Introduction
In mammals, the germ cell compartment is established early in embryonic development from the primordial germ cells (PGCs), the germline progenitor cells. PGCs arise outside of the gonad anlagen and they colonize the gonad by migration.Citation1 At the same time that this migration occurs, PGCs proliferate actively. By the time the embryonic gonad is fully colonized by PGCs [approximately 12.5 days post coitum (dpc) in the mouse] the population of PGCs is greatly increased.Citation1,Citation2 In male embryos, once the PGCs have reached the embryonic gonad, they stop proliferation and enter a quiescent period, being then referred to as gonocytes or prospermatogonia.Citation1 This happens at the same time as sexual differentiation occurs with the formation of testis cords in the male gonad.Citation3 In female embryos, PGCs enter directly into meiosis, giving rise to oocytes and are then arrested at the end of prophase of the first meiotic division.Citation4,Citation5 In males, gonocytes will resume mitosis a few days after birth [1 day post partum (dpp) in the mouse], giving rise to the spermatogonial stem cells of the adult testis (reviewed in refs. Citation3 and Citation6). At puberty, these cells will undergo meiosis and spermiogenesis generating mature sperm. In females, oocytes will resume meiosis in cohorts and give rise to mature eggs (reviewed in ref. Citation7).
In men, testicular cancer arises from carcinoma in situ cells, which are thought to derive from PGCs and prospermatogonia that have escaped normal differentiation. Human testicular cancers demonstrate consistent abnormalities in chromosome 12, such as the gain of isochromosome 12p, i(12p).Citation8 Several genes map to this region including cyclin D, the cyclin-dependent kinases (CDKs), CDK2, CDK4 and CDK6, Ras, p53 and MDM2,Citation9 and indeed some of these genes are deregulated in testicular cancer.Citation10–Citation14 In addition to those adult germ cell tumors that derive from PGCs, a less frequent adult germ cell tumor is the spermatocytic seminoma. The germ cell that is responsible for this tumor class has not been identified so far, but several data suggest that this type of adult germ cell tumor does not derive from PGCs but from a later stage, probably the spermatogonial stem cells (reviewed in ref. Citation15). So far, a murine model for this tumor type has not been established.
Cyclin E is an S phase specific activator of the cyclin-dependent kinase Cdk2, an important regulator of the cell cycle that drives the G1/S transition in higher eukaryotes.Citation16 Cyclin E protein levels peak near the G1/S boundaryCitation17 and decrease during S phase due to ubiquitin-mediated proteolysis controlled by the ubiquitin ligase SCFCdc4 [Skp1/Cdc53(cullin)/F-box protein-Cdc4].Citation18 Overexpression of cyclin E alone is sufficient to drive G1 cells into S phase,Citation19 inducing a new round of DNA synthesis. pRb has been established as a biologically relevant target of cyclin E-dependent kinase, but cyclin E promotes S-phase entry even in the absence of pRb,Citation20 suggesting the existence of other critical targets. During cell cycle re-entry from quiescence, cyclin E is required for the assembly of pre-replication complexes by promoting the accumulation of the kinase Ccd7 and the MCM loading factor Cdc6,Citation21–Citation23 which in turn is necessary for loading of pre-replication complex factors onto the chromatin and for initiation of DNA replication.
Cyclin E overexpression has been associated with cancer development and poor prognosis,Citation24–Citation27 even though the mechanisms underlying the oncogenic potential of cyclin E are still not completely understood. It has been thought that cyclin E might promote tumorigenesis by acting as a positive regulator of the cell cycle and stimulating proliferation (reviewed in ref. Citation28). Alternatively, it has been shown that cyclin E overexpression causes genomic instability,Citation29 a hallmark of cancer cells. This genomic instability would result in the loss of important tumor suppressors and/or the accumulation of deleterious oncogenes and therefore contribute to tumor development and progression.
Mice nullizygous for both known cyclin E isoforms, E1 and E2, have been generated and characterized.Citation22 Even though complete cyclin E deficiency leads to death during embryogenesis as a result of placental defects, single knock out animals are born at the expected frequencies. While cyclin E1 null mice appear completely normal, male mice deficient for cyclin E2 display reduced fertility due to abnormal spermatogenesis.Citation22 Interestingly however, cyclin E2-/- females develop normally and are fully fertile.
In order to try to better understand the possible role of cyclin E in the development and progression of germ cell tumors, we have generated transgenic mouse lines expressing a degradationresistant cyclin E mutant protein (cyclin ET62A, T380A) under the control of the Translation Elongation Factor 1alpha (EF-1alpha) promoter, which has been previously shown to target high protein expression specifically to dividing spermatogonia.Citation30,Citation31 Surprisingly, we found that cyclin ET62A, T380A males were not prone to germ cell malignancies, but instead had reduced fertility attributable to decreased numbers of germ cells in their seminiferous tubules.
Results
In order to analyze the effect of cyclin E deregulation in germ cells, we overexpressed cyclin ET62A, T380A, a mutant protein previously show to be resistant to degradation by the proteasome,Citation18 in mouse testes, by cloning it under the control of the EF1-alpha promoter (), previously shown to target high protein expression in the testicular germ cells.Citation30 Using Southern blot hybridization, we identified four transgenic founder animals (), of which #16 and #20 (both females) were used to establish transgenic lines. From the 3rd founder we never obtained transmission of the transgene to the progeny, while from the 4th one, the only male founder obtained, we were unable to obtain any progeny (). This suggests possible fertility problems with deregulated expression of cyclin E in male germ cells. From the two transgenic lines established, transgenic pups were born at approximately the expected Mendelian rate (see ) and developed normally to adulthood without obvious defects in any tissues other than testes. Aging studies were performed and the transgenic mice displayed a normal life span, with no increased predisposition to the development of testicular tumors.
Expression of the human transgenic cyclin ET62A, T380A was confirmed by immunohistochemistry in paraffin-embedded testicular sections, using an antibody raised against human cyclin E, that does not cross react with the endogenous murine protein. As can be seen in , high expression of human cyclin E can be observed in dividing spermatogonia, with expression decreasing substantially as the cells enter meiosis, in accordance with previously published expression patterns for EF1-α promoter transgenes.Citation30,Citation31 We then immunoprecipitated cyclin E from frozen mouse testes and measured the cyclin E-associated kinase activity, towards histone H1. As expected, cyclin E immunoprecipitates from transgenic mice showed increased kinase activity compared to wild-type littermates (), showing that the overexpressed cyclin E mutant is functional in the transgenic testes.
Upon dissection we observed that testes from cyclin ET62A,T380A transgenic mice appeared smaller than those of wild-type littermates, so we analyzed this phenotype in more detail. As can be observed in , testicular size is decreased in both transgenic lines, although with high variability, ranging from normal size to much smaller than those of wild type littermates. The observed differences are more drastic for transgenic line #16, in which the mutant cyclin E is expressed at higher levels. The more detailed phenotypic analysis described below was therefore performed in animals from this transgenic line.
In order to determine the cause of the decreased fertility of cyclin E transgenic males, testicular sections of adult testes were obtained. Spermatogenesis was defective in 50% to 80% of the seminiferous tubules, showing mostly Sertoli-cell-only (SCO) tubules, with no germ cells (compare and B). However, some of the seminiferous tubules showed complete spermatogenesis, demonstrating that meiosis and spermiogenesis could evade the effects of the cyclin E transgene in a subset of tubules ( and C). TUNEL analysis was performed in order to determine if germ cell apoptosis was the cause of reduced spermatogenesis. No significant difference was observed between cyclin E transgenic males and wild-type littermates (). These data indicate that the reduced fertility and decreased spermatogenesis in transgenic adult animals is due to improper seminiferous tubule colonization of germ cells. This could be due to improper mitosis in the embryonic PGCs, causing a defect in the seeding of gonocytes in the seminiferous tubules before birth or a defect in spermatogonial mitotic proliferation a few days after birth.
In order to ascertain which one of the possibilities mentioned above was correct, we scored the numbers of germ cells at 1 day post partum (day of birth) and quantified their division rate. The numbers of gonocytes colonizing the tubules of transgenic animals were slightly lower than wild type littermates, but this difference was not statistically significant (). These data indicate that there is normal survival and colonization of the seminiferous tubules of primordial germ cells in the embryonic gonads of the transgenic mice. In contrast, by ki67 immunostaining we found a statistically significant reduction in the number of proliferating germ cells in the transgenic testes at 1 dpp (). This result indicates that cyclin E overexpression impairs the ability of quiescent gonocytes to resume spermatogenetical cell division at 1 dpp, causing the improper germ cell/Sertoli cell ratio that in turn produces SCO tubules in the adult transgenic mice and hence the reduced fertility observed.
Discussion
In our study, the overexpression of cyclin E in spermatogonia did not cause testicular tumors. On the contrary, quiescent germ cells at 1 dpp were not able to re-enter mitotic divisions as evidenced by lower numbers of ki-67 positive germ cells at that stage in the testes of transgenic animals. This is probably due to accumulation of persistent non-degradable cyclin E in the embryonic germ cell during the week that they are quiescent (from approximately 13 dpc to 1 dpp). This may damage the cells and prevent them from re-entering the cell cycle after birth. Interestingly, it has been determined by qRT-PCR, that there is a striking reduction of cyclin E1 and E2 expression in 12.5–14.5 dpc mouse male germ cells during the early stages of entry into quiescence,Citation32 consistent with these cells being particularly sensitive to elevated levels of cyclin E at this time of development. Alternatively, high levels of cyclin E may interfere with germ cell proliferation directly after release from quiescence. It has been shown previously in other cell types that overexpression of cyclin E can inhibit both progression through S phase and the metaphase to anaphase transition.Citation33,Citation34
At this point, we cannot explain why some seminiferous tubules evade the effects of the cyclin E transgene and appear histologically normal. The most likely explanation is that the EF-1α promoter that drives cyclin E expression in our model can become epigenetically silenced in a subset of germ cells. Indeed, such silencing has been observed in other models that utilized this promoter (reviewed in ref. Citation30).
We infer that reduced spermatogonial proliferation in our transgenic animals in turn causes a reduced germ cell/Sertoli cell ratio, reduced spermatogenesis and male infertility. Other investigatorsCitation30 have made similar histological findings by overexpressing the anti-apoptotic factor Bcl-2. Transgenic expression of Bcl-2 in the male germ line first caused spermatogonial accumulation and later massive loss of germ cells. Ultimately, as in the cyclin E transgenics, male infertility was observed. Similar to our results, newborn testes were histologically normal and spermatogenesis was also partially recovered in some seminiferous tubules in which the level of Bcl-2 was reduced. The authors concluded that atypical spermatogonia, forced to survive by exogenous bcl-2 expression, might be at a disadvantage for meiotic differentiation. In the case of our transgenic males, the atypical accumulation of cyclin E in spermatogonia doesn't cause an initial increase in proliferation but instead prevents entry into mitotic cycles that normally occur prior to the meiotic divisions. Interestingly, neither model produced germ cell tumors.
Materials and Methods
Generation of transgenic mice.
Transgenic mice were generated by cloning the human cyclin ET62A, T380A cDNA under the control of the hEF1alpha promoter, as a BstXI fragment into the pEF-BOS vector (BCCM/LMBP Plasmid and DNA Library Collection). A 3.2 Kb HindIII/PvuI fragment containing the EF1alpha promoter, cyclin ET62A, T380A cDNA and a poly(A) adenylation signal was purified and microinjected into the pronuclei of fertilized mouse eggs (DBA/2 x C57BL/6). Integration of the transgene was detected by Southern blot hybridization of genomic DNA extracted from tail biopsies and digested with EcoRI, which releases the 1,250 bp cyclin ET62A, T380A cDNA, using the same cyclin ET62A, T380A cDNA as probe. After establishment of the mouse transgenic lines, further screening was performed by PCR, using a forward primer on the EF1α promoter and a reverse primer on the human cyclin E cDNA.
Histology, immunohystochemistry and immunocytochemistry.
Tissues from mice were harvested, weighed and fixed overnight in 10% neutral buffered formalin, routinely processed and embedded in paraffin. Tissues were sectioned to 6 µm and stained with hematoxylin and eosin by standard methods. For immunohistochemistry, paraffin-embedded sections were deparaffinized and rehydrated by xylene and a graded alcohol series. Antigen retrieval was performed by microwave in Vector Labs retrieval solution (pH 6.0; BD Pharmigen). Antibody staining and counterstaining with hematoxylin was performed as described.Citation35 Primary antibodies used were: human cyclin E (Santa Cruz Biotechnologies, 1:500), PCNA (Santa Cruz Biotechnologies, 1:1,000) and Ki67 (Abcam, 1:20). Apoptotic cells were detected using the TUNEL staining Kit (Roche), according to the manufacturer's instructions.
Immunoblotting and in vitro kinase assays.
Protein extracts were extracted from frozen tissues as described,Citation35 and western blotting was performed by standard techniques. Human HEK293A cells were transfected with different cyclin E constructs, using Lipofectamine (Invitrogen) according to the manufacturer's instructions. Cells were harvested and lysed in lysis buffer (50 mM Tris, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100 and Complete protease inhibitors) (Roche) and cleared by centrifugation at 10,000 G for 15 minutes at 4°C. Protein concentration was measured using Bradford protein assay (Bio-Rad). For in vitro kinase assay, cyclin E/Cdk2 complexes were immunoprecipitated from extracts prepared from frozen tissues or 293A cells by incubating overnight in lysis buffer with an antibody specific for human cyclin E, that does not react with the mouse cyclin E protein (HE172, Santa Cruz Biotechnologies). Immunoprecipitated proteins were washed 3 times with lysis buffer and incubated with 5 µg of histone H1 for 30 minutes at 37°C in kinase buffer (20 mM Tris-HCl pH 7.5, 15 mM MgCl2 10 µM ATP and 10 µCi (gamma-32P) ATP. Kinase reactions were stopped by addition of SDS sample buffer and samples were run on a 12% SDS PAGE gel. The gel was dried and incorporation of (gamma-32P) ATP was analyzed by autoradiography.
Ethics.
Investigations involving animals were conducted in accordance with the National Research Council (NRC) publication Guide for Care and Use of Laboratory Animals (copyright 1996, National Academy of Science).
Figures and Tables
Figure 1 Generation of transgenic mice expressing a cyclin E mutant (Cyclin ET62A, T380A) under control of the hEF1-alpha promoter. (A) Targeting vector for generation of transgenic mice expressing human cyclin ET62A, T380A in testicular germ cells, under the control of the hEF1-alpha promoter. (B) Screening, by Southern blot hybridization, of tail genomic DNA digested with EcoRI and probed with human Cyclin E cDNA. Single band of approximately 1,250 bp identifies mice that have incorporated the transgene. Plasmid DNA containing the targeting construct was digested with EcoRI and used as positive control.
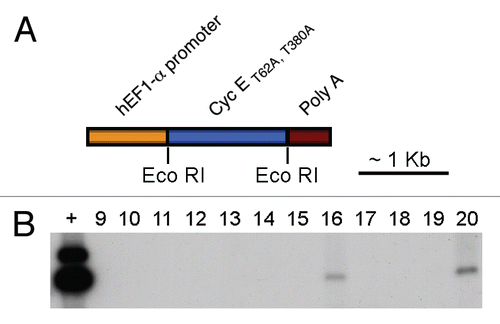
Figure 2 Exogenous cyclin E is expressed in testicular germ cells and increases Cdk2 kinase activity. (A–C) Detection, by immunohistochemistry on mouse testis sections, of human cyclin E expression (brown nuclei) in transgenic mice (B and C) and wild-type (A) littermates (A, C: 630x; B: 400x). (D) In vitro kinase activity toward histone H1 of human cyclin E immunoprecipitates from hcyclin ET62A, T380A transgenics or wild-type littermates.
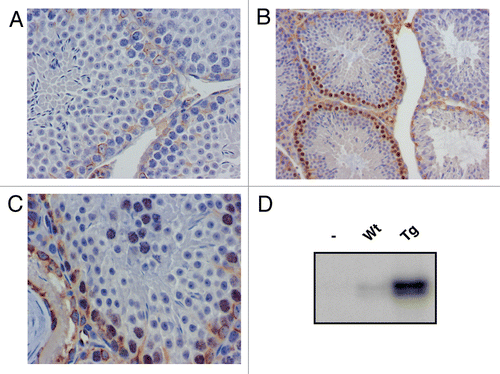
Figure 3 Spermatogenesis is defective in 50–80% of the seminiferous tubules of transgenic mice. (A) H&E staining in wild type adult mouse testicular sections, showing complete spermatogenesis in all tubules (100x). (B) Cyclin E transgenic adult mouse testis section showing most seminiferous tubules lacking germ cells and a few with complete spermatogenesis (100x). (C) Complete spermatogenesis in transgenic mice, demonstrating that meiosis and spermiogenesis is unaffected in the transgenic animals.
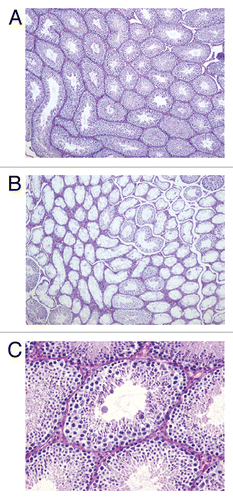
Figure 4 Tunnel staining shows no difference in apoptosis in cyclin E transgenic and wild type littermates. (A and B) Detection of apoptotic cells by TUNEL staining (brown nuclei) of 12.5 day post partum (dpp) mouse testicular sections from wild type (A) and cyclin E transgenic animals (B) (100x).
Figure 5 Cyclin E transgenic overexpression impairs the ability of quiescent gonocytes to resume spermatogenetical mitosis. (A) Number of gonocytes per cross-sectioned tubule in 1 dpp testicular sections. No statistical differences were found (Student's t-test). Data are indicated as mean and error bars represent standard deviation (SD). (B) Number of ki-67 positive (dividing) gonocytes per cross-sectioned tubule in testicular sections of wild type and cyclin E transgenics at 1 dpp. Statistical differences were observed (Student's t-test). Data are indicated as mean and SD.
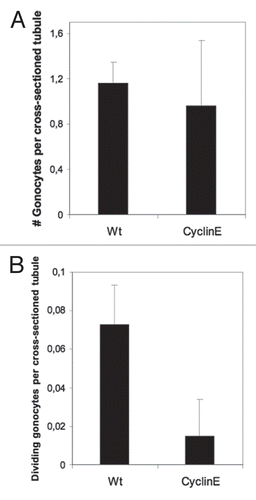
Table 1 Generation of transgenic mice expressing a stabilized cyclin E mutant, under control of the hEF1-alpha promoter
Table 2 Testicular atrophy in cyclin ET62A, T380A transgenic mice
Acknowledgements
This work was supported by grants from Lance Armstrong Foundation (V.L.), NIH/NCI grant CA078343 (S.I.R.) and SAF2008-03837 from Ministry of Science and Innovation and Agencia Laín Entralgo, Madrid, Spain (M.P.M.).
References
- McLaren A. Germ Cells and Soma: A new look at an old problem 1981; New Haven, CT Yale University Press
- Tam PP, Snow MH. Proliferation and migration of primordial germ cells during compensatory growth in mouse embryos. J Embryol Exp Morphol 1981; 64:133 - 147
- De Rooij DG. Regulation of the proliferation of spermatogonial stem cells. J Cell Sci Suppl 1988; 10:181 - 194
- Borum K. Oogenesis in the mouse. A study of the meiotic prophase. Exp Cell Res 1961; 24:495 - 507
- Speed RM. Meiosis in the foetal mouse ovary. I. An analysis at the light microscope level using surface-spreading. Chromosoma 1982; 85:427 - 437
- De Rooij DG, Van Dissel-Emiliani FM, Van Pelt AM. Regulation of spermatogonial proliferation. Ann NY Acad Sci 1989; 564:140 - 153
- Lin H. The tao of stem cells in the germline. Annu Rev Genet 1997; 31:455 - 491
- Looijenga LH, Zafarana G, Grygalewicz B, Summersgill B, Debiec-Rychter M, Veltman J, et al. Role of gain of 12p in germ cell tumour development. APMIS 2003; 111:161 - 171
- Delozier-Blanchet CD, Engel E, Walt H. Isochromosome 12p in malignant testicular tumors. Cancer Genet Cytogenet 1985; 15:375 - 376
- Riou G, Barrois M, Prost S, Terrier MJ, Theodore C, Levine AJ. The p53 and mdm-2 genes in human testicular germ-cell tumors. Mol Carcinog 1995; 12:124 - 131
- Sicinski P, Donaher JL, Geng Y, Parker SB, Gardner H, Park MY, et al. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature 1996; 384:470 - 474
- Eid H, Institoris E, Geczi L, Bodrogi I, Bak M. mdm-2 expression in human testicular germ-cell tumors and its clinical value. Anticancer Res 1999; 19:3485 - 3490
- Roelofs H, Mostert MC, Pompe K, Zafarana G, van Oorschot M, van Gurp RJ, et al. Restricted 12p amplification and RAS mutation in human germ cell tumors of the adult testis. Am J Pathol 2000; 157:1155 - 1166
- Schmidt BA, Rose A, Steinhoff C, Strohmeyer T, Hartmann M, Ackermann R. Upregulation of cyclin-dependent kinase 4/cyclin D2 expression but downregulation of cyclin-dependent kinase 2/cyclin E in testicular germ cell tumors. Cancer Res 2001; 61:4214 - 4221
- Oosterhuis JW, Looijenga LH. Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer 2005; 5:210 - 222
- Resnitzky D, Gossen M, Bujard H, Reed SI. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol 1994; 14:1669 - 1679
- Dulic V, Lees E, Reed SI. Association of human cyclin E with a periodic G1-S phase protein kinase. Science 1992; 257:1958 - 1961
- Strohmaier H, Spruck CH, Kaiser P, Won KA, Sangfelt O, Reed SI. Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature 2001; 413:316 - 322
- Resnitzky D, Reed SI. Different roles for cyclins D1 and E in regulation of the G1-to-S transition. Mol Cell Biol 1995; 15:3463 - 3469
- Lukas J, Herzinger T, Hansen K, Moroni MC, Resnitzky D, Helin K, et al. Cyclin E-induced S phase without activation of the pRb/E2F pathway. Genes Dev 1997; 11:1479 - 1492
- Masai H, Matsui E, You Z, Ishimi Y, Tamai K, Arai K. Human Cdc7-related kinase complex. In vitro phosphorylation of MCM by concerted actions of Cdks and Cdc7 and that of a criticial threonine residue of Cdc7 bY Cdks. J Biol Chem 2000; 275:29042 - 29052
- Geng Y, Yu Q, Sicinska E, Das M, Schneider JE, Bhattacharya S, et al. Cyclin E ablation in the mouse. Cell 2003; 114:431 - 443
- Chuang LC, Teixeira LK, Wohlschlegel JA, Henze M, Yates JR, Mendez J, et al. Phosphorylation of Mcm2 by Cdc7 promotes pre-replication complex assembly during cell cycle re-entry. Mol Cell 2009; 35:206 - 216
- Keyomarsi K, Pardee AB. Redundant cyclin overexpression and gene amplification in breast cancer cells. Proc Natl Acad Sci USA 1993; 90:1112 - 1116
- Bortner DM, Rosenberg MP. Induction of mammary gland hyperplasia and carcinomas in transgenic mice expressing human cyclin E. Mol Cell Biol 1997; 17:453 - 459
- Sakaguchi T, Watanabe A, Sawada H, Yamada Y, Yamashita J, Matsuda M, et al. Prognostic value of cyclin E and p53 expression in gastric carcinoma. Cancer 1998; 82:1238 - 1243
- Datta MW, Renshaw AA, Dutta A, Hoffman MA, Loughlin KR. Evaluation of cyclin expression in testicular germ cell tumors: cyclin E correlates with tumor type, advanced clinical stage and pulmonary metastasis. Mod Pathol 2000; 13:667 - 672
- Berglund P, Landberg G. Cyclin e overexpression reduces infiltrative growth in breast cancer: yet another link between proliferation control and tumor invasion. Cell Cycle 2006; 5:606 - 609
- Spruck CH, Won KA, Reed SI. Deregulated cyclin E induces chromosome instability. Nature 1999; 401:297 - 300
- Furuchi T, Masuko K, Nishimune Y, Obinata M, Matsui Y. Inhibition of testicular germ cell apoptosis and differentiation in mice misexpressing Bcl-2 in spermatogonia. Development 1996; 122:1703 - 1709
- Meng X, Lindahl M, Hyvonen ME, Parvinen M, de Rooij DG, Hess MW, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 2000; 287:1489 - 1493
- Western PS, Miles DC, van den Bergen JA, Burton M, Sinclair AH. Dynamic regulation of mitotic arrest in fetal male germ cells. Stem Cells 2008; 26:339 - 347
- Ekholm SV, Reed SI. Regulation of G(1) cyclin-dependent kinases in the mammalian cell cycle. Curr Opin Cell Biol 2000; 12:676 - 684
- Keck JM, Summers MK, Tedesco D, Ekholm-Reed S, Chuang LC, Jackson PK, et al. Cyclin E overexpression impairs progression through mitosis by inhibiting APC(Cdh1). J Cell Biol 2007; 178:371 - 385
- Smith AP, Henze M, Lee JA, Osborn KG, Keck JM, Tedesco D, et al. Deregulated cyclin E promotes p53 loss of heterozygosity and tumorigenesis in the mouse mammary gland. Oncogene 2006; 25:7245 - 7259