Abstract
Weakly electric fish perceive their actively generated electrical field with cutaneous electroreceptors. This active sensory system is used both for orientation and for communication. In a recent paper1 we focussed on how anatomical adaptations (pre-receptor mechanisms), biophysical constraints and behavior all contribute to active electrolocation, i.e. the fishes’ unique ability to determine and distinguish the electrical properties of objects based on the modulation of a self-generated carrier signal, the so-called electric organ discharge.
How weakly electric fish are able to use the information contained in their own signals has been the centre of many investigations. Several approaches, ranging from psychophysics,Citation2 modellingCitation3–Citation5 and anatomyCitation6 to neurophysiologyCitation7–Citation10 have focused on one key question: what information about an object can fish extract by use of the electrical images? The electric image is the area on the fish's sensory surface that is influenced by an object under investigation during active electrolocation. The abundance of approaches pursued reflects the difficulty of measuring and quantifying electrical images. Here we extend on our recent resultsCitation1 of active electrolocation in Gnathonemus petersii (Günther 1862) and set the stage for a generalisation of problems in electrical imaging.
In essence, information in electrical images is contained in a 2-dimensional distribution of amplitude-modulations of the EOD. Due to a lack of a focussing mechanism (e.g., in vision this would be the lens of the eye), electrical images can be ambiguous. Thus, the shape of an electrical image depends on several object properties and on the distance between the object and the animal (). Additionally, a minimal extend of an electrical image must be projected onto the sensory surface containing the electroreceptor organs (which are embedded in the animals' skin) in order to extract enough information on object properties. These limitations explain why the electric sense is a near-field sensory system, since far-away objects project incomplete electrical images of reduced amplitude on the sensory surface (see ).
Our experimentsCitation1,Citation6,Citation11 as well as those of other authors on other electrical fish speciesCitation12,Citation13 have shown that electroreceptor organs occur with highest densities in the peri-oral region, and, in the case of Gnathonemus, especially on the moveable chin appendage, the so-called Schnauzenorgan. In contrast, the density of electroreceptors on the trunk is lower by at least one order of magnitude. These anatomical differences as well as the observation of unique motor patterns and our measurements of the local EOD propertiesCitation1 (), support the hypothesisCitation14 that there exist two electrosensory foveae in G. petersii: the Schnauzenorgan with its extreme density of electroreceptors and its flexibility is thought to be a fovea used for close-range imaging during foraging; whereas the so-called nasal region (the skin region between the mouth and the nares) might be highly suitable for a detailed analysis of objects in the wider range of active electrolocation.
This idea of two foveae is supported by the body positioning during foraging, when the nasal fovea points forward to perceive approaching, far away objects, while the Schnauzenorgan fovea points downwards and is passed in an oscillating sweeping movement over the ground in order to search for and identify prey items.Citation11 This focusing of the senses towards the ground has also been found for the eyes of Gnathonemus,Citation15 where the region of highest ganglion-cell density is also oriented towards the bottom.
While the spatial acuity of electrolocation is expected to be maximal at the foveal regions, the Schnauzenorgan and head are only exposed to a small aspect of an electrical image, especially when the objects are comparatively large, such as those that were used during recent behavioral discrimination experiments.Citation16 A sensory surface more suitable for the projection of larger electrical images might be the trunk. However, here the spatial acuity is smaller due to the lower electroreceptor density.Citation11 Physiological data indicate that the trunk, albeit its low receptor density, might be involved in movement/contour-detection.Citation17
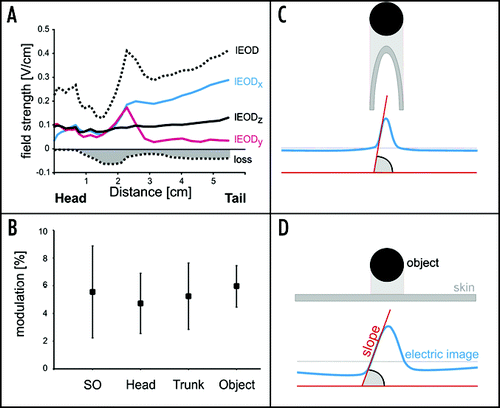
In addition to the geometrically different shapes of the foveae, the changing properties of the local EOD (lEOD) are another aspect that can lead to ambiguities during active electrolocation. This can be illustrated when the lEOD is divided into three vectorial components, representing the three-spatial axis. While the vectorial components of the EOD are constant and in phase at the head region, they vary at the trunk: the medio-lateral (x-) component increases from head to trunk, while the rostro-caudal (y-) component decreases (). In addition, the three EOD-components are out of phase at the trunk. This results in a decrease in coherence of the EOD (). Thus, body shape and local EOD properties result in differences of electrical images at different body regions as is summarised in C and D. Hence, in order for the fish to make sense of the information contained in an electrical image, it must take into account which part of the body the image is projected on.
Recent physiological results suggest that the neuronal representation of the trunk sensory surface at the first level of central processing might be optimal for the processing of contours and of movements.Citation8,Citation17 This is in line with modelling data which suggest that the trunk might be the body region suited best for the discrimination between neighbouring objects.Citation4 Together with our data this strongly suggests that the distribution of the sensory organs and their central representation as well as the constraints for accurate electrical imaging have lead to a functional segregation of different body region, in particular of the two rostral foveae and the trunk.
Our dataCitation1 show that in Gnathonemus the electrosensory fovea at the Schnauzenorgan is unique due to pre-receptor mechanisms which funnel the self-generated current towards its tip. We expect that similar effects are also present in other Mormyrid species, where the highest densities of electroreceptors are also found at the chin.Citation11 Such protuberances are likely to funnel the currents onto the presumed perioral fovea.Citation12 Gnathonemus is unique, however, since it actively moves the finger-like Schnauzenorgan in search of food. There are many examples where motion constitutes re-afferent interference during sensory processing,Citation18–Citation22 and electric fish have been most valuable in establishing how corollary discharge information can be used to cancel such interferences.Citation21,Citation22 Our data on the fovea of Gnathonemus adds a yet unprecedented example for a passive suppression of such re-afferent signals. We found that bending of the Schnauzenorgan did not alter the electrical field at its tip.Citation1 Hence the fovea at the Schnauzenorgan can be moved without adding additional ambiguity. This is contrary to swimming movements, which shift the source of the EOD, the electric organ. This leads to modulations of the local EOD at all body regions (), and these modulations equal those caused by nearby objects. Several studies have shown that corollary discharge mechanisms are employed by the fish to cancel such re-afferent effects. However, convincing examples are still lacking of how the mechanisms proposedCitation10,Citation22 (negative image formation due to synaptic plasticity) can work when the re-afference changes on an EOD-to-EOD timescale.
One general issue emerging from recent models of active sensingCitation3,Citation4 is the contribution of correlations of spatial and temporal aspects to the prevention of ambiguities during sensory imaging. Such correlations occur whenever there is relative motion between the fish and an object. In analogy to the visual system,Citation23,Citation24 we refer to this as the electrical flow. Recent experiments in Gymnotus omariCitation25 and Gnathonemus petersiiCitation8,Citation10 have shown that changes in electric images are evaluated with respect to a previous baseline. Thus, changes in electrical flow are detectable and this should enable the animals to perceive spatiotemporal correlations in the electrical flow. The region probably best suited for this is the trunk, where the electroreceptors occur at low density, but their central physiology suggests that this region might be well suited for motion detection.Citation17 The foveal regions at the head, in contrast, appear to be more appropriate for accurate detection and discrimination of nearby objects.
Figures and Tables
Figure 1 The simplest form of an electric image simulated by an electrical dipole which is equivalent to a small conductive sphere.Citation26 If projected on a plane surface, the current density depends on the object's distance to the surface and on its size. The 2-D distribution of the current density is shown for a small sphere located at 1 cm (dotted line and upper image) and 1.5 cm (solid line and lower image) from the skin together with a transect through the centres of both images. Note that both peak and widths of the images are influenced by distance. The inset on the left shows an exemplary EOD of Gnathonemus petersii.
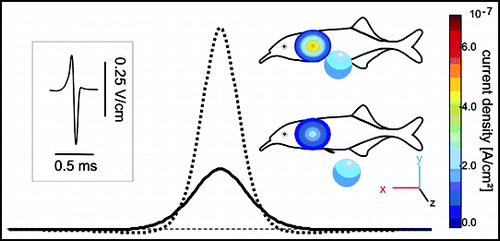
Figure 2 Properties of the local electric field (A and B) and the influence of body shape on electrical images (C and D). (A) Distribution of the three components of the local EOD (x, y and z). The summed amplitude is shown by the dotted line. The head is characterised by lEODs that are either completely in phase. In contrast, the local EOD at the trunk is less coherent. This is reflected in a loss of field strength of the lEOD as shown by the dotted line and filled area (‘loss’). (B) Modulation of the local EOD by passively bending the tail ipsilaterally by 30°. In contrast to motion of the chin appendage alone, the movement of the tail (and thus the electrical source) leads to a constant increase of the signal carrier amplitude. This modulation is in the same range as that caused by nearby objects, i.e., electrical images and re-afferent modulations can be equal in amplitude. (C and D) Schematic illustrations of the influence of the shape of the sensory surface on the electrical images projected onto them. A spherical object facing a curved sensory surface (C), for example the Schnauzenorgan, leads to an electrical image of a comparatively pointed contour. A spherical object opposite a flat sensory surface, in contrast, leads to a smoother and widened electrical image (D).
Addendum to:
References
- Pusch R, von der Emde G, Hollmann M, Bacelo J, Nöbel S, Grant K, Engelmann J. Active sensing in a mormyrid fish: electric images and peripheral modifications of the signal carrier give evidence of dual foveation. J Exp Biol 2008; 211:921 - 934
- von der Emde G, Schwarz S, Gomez L, Budelli R, Grant K. Electric fish measure distance in the dark. Nature 1998; 395:890 - 894
- Babineau D, Longtin A, Lewis JE. Modeling the electric field of weakly electric fish. J Exp Biol 2006; 209:3636 - 3651
- Babineau D, Lewis JE, Longtin A. Spatial acuity and prey detection in weakly electric fish. PLoS Comp Biol 2007; 3:38
- Migliaro A, Caputi AA, Budelli R. Theoretical analysis of pre-receptor image conditioning in weakly electric fish. PLoS Comp Biol 2005; 1:123 - 131
- Bacelo J, Engelmann J, Hollmann M, von der Emde G, Grant K. Peripheral distribution and central representation of electroreceptors in Gnathonemus petersii indicate functional foveae. J Comp Neurol 2008; In Press
- Gomez L, Budelli R, Grant K, Caputi AA. Pre-receptor profile of sensory images and primary afferent neuronal representation in the mormyrid electrosensory system. J Exp Biol 2004; 207:2443 - 2453
- Engelmann J, Bacelo J, Metzen M, Pusch R, Bouton B, Migliaro A, Caputi A, Budelli R, Grant K, von der Emde G. Electric imaging through active electrolocation: implication for the analysis of complex scenes. Biol Cybern 2008; 98:519 - 539
- Lewis JE, Maler L. Neuronal population codes and the perception of object distance in weakly electric fish. J Neurosci 2001; 21:2842 - 2850
- Sawtell NB, Williams A. Transformations of electrosensory encoding associated with an adaptive filter. J Neurosci 2008; 28:1589 - 1612
- Hollmann M, Engelmann J, von der Emde G. Distribution, density and morphology of electroreceptor organs in mormyrid weakly electric fish: anatomical investigations of a receptor mosaic. J Zool 2008; http://dx.doi.org/10.1111/j.1469-7998.2008.00465.x
- Castelló ME, Aguilera PA, Trujillo-Cenoz O, Caputi AA. Electroreception in Gymnotus carapo: pre-receptor processing and the distribution of electroreceptor types. J Exp Biol 2000; 203:3279 - 3287
- Shumway CA. Multiple electrosensory maps in the medulla of weakly electric gymnotiform fish II. Anatomical differences. J Neurosci 1989; 9:4400 - 4415
- von der Emde G, Schwarz S. How the electric fish brain controls the production and analysis of electric signals during active electrolocation. Zoology 2001; 103:112 - 114
- Wagner HJ. Bipolar cells in the “grouped retina” of the elephantnose fish (Gnathonemus petersii). Vis Neurosci 2007; 24:355 - 362
- von der Emde G, Fetz S. Distance, shape and more: recognition of object features during active electrolocation in a weakly electric fish. J Exp Biol 2007; 210:3082 - 3095
- Metzten M, Engelmann J, von der Emde G. Receptive field properties of neurones in the electrosensory lateral line lobe of the weakly electric fish, Gnathonemus petersii. J Comp Physiol A accepted
- Bell C, Bodznick D, Montgomery J, Bastian J. The generation and subtraction of sensory expectations within cerebellum-like structures. Brain Behav Evol 1997; 50:17 - 31
- Oertel D, Young ED. What's a cerebellar circuit doing in the auditory system?. Trends Neurosci 2004; 27:104 - 110
- Kern R, van Hateren JH, Egelhaaf M. Representation of behaviorally relevant information by blowfly motion-sensitive visual interneurons requires precise compensatory head movements. J Exp Biol 2006; 209:1251 - 1260
- Bastian J. Plasticity of feedback inputs in the apteronotid electrosensory system. J Exp Biol 1999; 202:1327 - 1337
- Sawtell NB, Williams A, Bell CC. From sparks to spikes: information processing in the electrosensory systems of fish. Curr Opin Neurobiol 2005; 15:437 - 443
- Egelhaaf M, Boddeker N, Kern R, Kretzberg J, Lindemann JP, Warzecha AK. Visually guided orientation in flies: case studies in computational neuroethology. J Comp Physiol A 2003; 189:401 - 409
- Karmeier K, van Hateren JH, Kern R, Egelhaaf M. Encoding of naturalistic optic flow by a population of blowfly motion-sensitive neurons. J Neurophysiol 2006; 96:1602 - 1614
- Caputi AA, Aguilera PA, Castellø ME. Probability and amplitude of novelty responses as a function of the change in contrast of the reafferent image in G carapo. J Exp Biol 2003; 206:999 - 1010
- Sicardi EA, Caputi AA, Budelli R. Physical basis of distance discrimination in weakly electric fish. Physica A 2000; 86 - 93