Abstract
Proper transport and positioning of cell organelles often depends on the antagonistic activities of dynein and kinesin-1, two microtubule motors with opposite directionality.1 One of the largest known transport cargoes is the cell nucleus. Both dynein and kinesin-1 participate in positioning of the nucleus through binding to the nuclear envelope (NE).2-9 Surprisingly, both dynein and kinesin-1 can be recruited to the NE through multiple pathways, one involving SUN-KASH domain containing proteins and the other involving nuclear pore complexes (NPCs). Here, we discuss the molecular mechanisms of dynein and kinesin recruitment to the NE through NPCs, as well as the functional implications of dynein and kinesin-1 activity at the NE in mammalian cells. Finally, we discuss how motor activities at the NE might be controlled during the cell cycle.
Introduction
The cell nucleus is a very large organelle, and therefore its correct positioning requires a substantial amount of force. Many examples of active repositioning of the nucleus have been described. For example, after fertilization in C. elegans oocytes, the male and female pronucleus migrate towards each other and subsequently towards the center of the oocyte where they fuse to reconstitute a diploid genome. In budding yeast, the nucleus must migrate into the bud neck during mitosis to ensure that both the mother and daughter cell receives a complete set of the genetic material when the chromosomes segregate during anaphase. Also in filamentous fungi nuclear migration is an important process, which ensures even nuclear spacing during hyphal growth.Citation10 In mammals, newly born neuronal cells often need to migrate enormous distances during development of the brain to reach their final destination. During the process of neuronal migration, the nucleus must migrate through the cell body towards to leading edge of the migrating neuron (reviewed in ref. Citation11–Citation14). Thus, it is clear that active nuclear positioning is a common process required for many aspects of cell and tissue growth, development and homeostasis.
Bi-Directional Transport of the Nucleus
Several types of cytoskeletal components are known to control movement and positioning of the nucleus, including actin, intermediate filaments as well as microtubules. Here, we focus on the role of microtubule-based processes in nuclear positioning, as the roles of actin and intermediate filaments have been reviewed in reference Citation15. The minus-end directed motor dynein has been identified as a critical regulator of microtubule-dependent nuclear positioning in many different cellular systems. Dynein is thought to act in two ways during nuclear positioning. First, dynein localizes to the nuclear envelope in a variety of organisms.Citation3,Citation5,Citation16 At the nuclear envelope, dynein is thought to walk along microtubules towards their minus-ends. Since the microtubule minus-ends are embedded in the centrosome, minus-end directed motility of dynein results in a pulling force that brings the nucleus and centrosome towards each other. In this manner, continuous activity of dynein can ensure that centrosomes remain closely associated with the nucleus. This type of motility is, in fact, analogous to vesicle transport, in which dynein is docked on a vesicle and transports the vesicle towards the minus-ends of the microtubule. Second, dynein also localizes to the cortex, where it is thought to pull on centrosomal microtubules.Citation17 As the centrosome is either embedded in the nuclear envelope (as in budding yeast), statically attached to it (like in C. elegans embryoCitation18) or mechanically coupled to the nucleus through dynein activity at the nuclear envelope (as outlined above), displacement of the centrosome could also result in displacement of the nucleus. Indeed, loss of dynein function results in dramatic mis-positioning of the nucleus in many model organisms (reviewed in ref. Citation19). Similarly, in human cells, inhibition of dynein results in movement of the nucleus into the corner of the cell.Citation6,Citation20 In addition to nuclear mis-positioning, dynein inhibition also results in detachment of the centrosomes from the nucleus in flies, worms and human cells.Citation3,Citation5,Citation6
Strikingly, in human cells acute inhibition of dynein by function-blocking antibody injection results in almost immediate separation of the nucleus and centrosomes and rapid movement of these structures towards opposite corners of the cell.Citation6 This suggests that separation of nucleus and centrosomes is driven by active transport, rather than passive diffusion. Indeed, inhibition of the microtubule motor kinesin-1, a plus-end directed motor that antagonizes many aspects of dynein function, eliminates the extreme separation of centrosomes and nucleus,Citation6 suggesting that kinesin-1 is responsible for the force that pushes centrosomes and the nucleus apart.
How could kinesin-1 motors push centrosomes and the nucleus apart? The most likely explanation is that kinesin-1 binds to the nuclear envelope and attempts to transport the entire nucleus towards the plus-ends of centrosomal microtubules, as it does with many cellular organelles (). However, in contrast to other organelles, the nucleus is so large that kinesin-1 not only transports it to the plus-ends of microtubules, like it would do when transporting a vesicle, but also pushes the entire micro-tubule network away from the nucleus (). In normal cells, such extreme movements of nucleus and microtubule network is prevented by the activity of dynein, which continuously draws the nucleus and centrosomes back together. Consistent with this, overexpression of Nesprin 4, a nuclear envelope protein that binds kinesin-1 and might link kinesin-1 to the nuclear envelope, results in excessive separation of centrosomes and the nucleus and this effect depends on the binding of Nesprin 4 to kinesin-1.Citation9 These results suggest that kinesin-1 binding to the nucleus can generate a force that pushes centrosomes and the nucleus apart. Taken together, it is likely that bidirectional transport by microtubule motors of opposite polarity controls the relative position of the nucleus and microtubule cytoskeleton.
Molecular Targeting of Microtubule Motors to the Nuclear Envelope through Nuclear Pore Components
Two distinct pathways have been identified that link microtubule motors to the nuclear envelope. The first and best studied link consists of SUN-KASH domain proteins, which span the nuclear membranes to directly link the motors to the nuclear lamina. Their function in nuclear positioning has been covered in an excellent recent review in reference Citation12. In addition, both kinesin-1 and dynein can associate with the nuclear envelope through interaction with nuclear pore components.Citation6,Citation21,Citation22 Dynein associates with nuclear pore complex protein RanBP2 through its conserved adaptor protein Bicaudal D2 (BICD2), which directly interacts with RanBP2.Citation6 Kinesin-1 can also directly bind to RanBP2, and, in addition, kinesin-1 can associate with BICD2 as well.Citation21,Citation23 It is currently unclear whether the predominant interaction between kinesin-1 and RanBP2 is direct, or rather through BICD2. There is however, clear evidence that BICD2 plays a central role in kinesin-1 function at the nuclear envelope, as depletion of BICD2 prevents the kinesin-1-dependent separation of centrosomes and nucleus in cells lacking dynein activity.Citation6 RanBP2, on the other hand, was shown to enhance the ATPase activity of kinesin-1 in vitro,Citation24 suggesting that it might control kinesin-1 activity in addition to its localization at the nuclear envelope. To further complicate matters, the C. elegans homolog of the human Bicaudal D proteins, BICD-1 also binds to the nuclear envelope, but rather than binding to the nuclear pore component RanBP2, it associates with the KASH-domain containing protein Unc-83.Citation25 It is unclear whether the difference in Bicaudal D targeting to the nuclear envelope in these two studies, is due to a difference between species, or whether Bicaudal D in fact has two independent binding sites in the nuclear envelope. Importantly, participation in nuclear movements is an evolutionary conserved function of Bicaudal D, as it is also required for nuclear translocation and positioning in flies.Citation26,Citation27 In addition to Bicaudal D, other dynein co-factors such as Lis1 and Nde1/Ndel1 might also be involved for efficient nuclear envelope targeting and/or force generation by cytoplasmic dynein,Citation28 and it seems likely that a large protein complex involving multiple protein-protein interactions is in fact assembled on the nuclear surface to ensure effective positioning or motility of the nucleus.
Taken together, it is clear that the targeting of both dynein and kinesin-1 to the nuclear envelope is very complex and probably involves multiple parallel pathways that control not only the recruitment of these motors to the nuclear envelope, but also their activity. This is analogous to dynein targeting to kinetochores in mitosis which also appears to involve multiple parallel pathways.Citation29
Cell Cycle-dependent Changes in the Forces Acting on the Nucleus
While it is clear that microtubule motors generate forces to ensure correct positioning of the nucleus relative to the microtubule cytoskeleton, much less is known about the cell cycle-dependent changes in the forces that act on the nucleus. In G2, several hours before cells enter mitosis, BICD2 switches its localization from Rab6-positive vesicles to RanBP2 in the nuclear envelope.Citation6 This switch in BICD2 localization likely triggers, at least in part, dynein recruitment to the nuclear envelope, which is also known to occur at this time.Citation30 It is however, currently unknown what induces the sudden recruitment of BICD2 to the nuclear envelope. Perhaps phosphorylation of BICD2 or RanBP2 by a cell cycle-regulated kinase triggers the switch in BICD2 localization, but this needs to be further investigated. It is likely that G2 specific recruitment of dynein to the nuclear envelope has a major impact on the forces acting on the nucleus. This idea is supported by the fact that cytoplasmic nuclear pore complexes (known as annulate lamellae) that also recruit BICD2 and dynein specifically in G2, start to move towards the minus-ends of microtubules at the exact time when BICD2 and dynein are recruited.Citation6 Thus, it is likely that recruitment of BICD2 and dynein to the nuclear envelope ensures tight coupling of the centrosomes to the nucleus at this stage.
An additional example of specificity in temporal recruitment of dynein to the nuclear envelope has been found in a study of early C. elegans development. After fertilization, dynein was reported to localize specifically to the female, but not the male pronucleus,Citation22 while in later divisions, dynein appears to localize to the nuclear envelope of every cell.Citation18 Interestingly, in this system dynein was suggested to interact with nuclear pore components as well on the nuclear envelopes of the female pronucleus,Citation22 although additional pathways involving SUN-KASH domain containing proteins likely contribute to dynein recruitment to the nuclear envelope in these cells as well.Citation18 Thus the exact function of dynein at the nuclear envelope is likely different in different cell types (for example, dynein has also been implicated in meiotic chromosome pairing in C. elegansCitation31), which requires that recruitment of dynein to the nuclear envelope is a tightly regulated process.
While dynein has been directly visualized at the nuclear envelope, this has proven more difficult for kinesin-1, probably due to the high level of diffuse cytoplasmic protein.Citation6,Citation7 Therefore, it is currently unclear if and when kinesin-1 is recruited to the nuclear envelope. However, one piece of indirect evidence is available; when dynein is depleted from cells, the centrosomes stay closely associated with the nuclear envelope throughout a large part of the cell cycle, but strikingly, ∼1 h before cells enter mitosis, the centrosomes and the nucleus rapidly move apart,Citation6 suggesting that at this time kinesin-1 is recruited to or, alternatively activated at the nuclear envelope. These results indicate that the forces acting on the nuclear envelope are tightly linked to cell cycle progression and suggest that dynein and kinesin might have a specific role at the nuclear envelope around the time of mitotic entry.
It is interesting to note that these cell cycle-dependent changes in the recruitment of dynein and kinesin-1 to the nucleus appear to be mediated specifically through the interaction of dynein and kinesin-1 with RanBP2. As mentioned before, both dynein and kinesin-1 also associate with the nuclear envelope through SUN-KASH domain containing proteins,Citation12,Citation15 so it will be interesting to know whether these interactions are also coupled to the cell cycle. Alternatively, the cell cycle-related function of dynein and kinesin-1 at the nuclear envelope might be mediated mostly through RanBP2, while the recruitment of these motors during other processes might be predominantly mediated through SUN-KASH proteins. This will be an important aspect of future research.
Function of Kinesin-1 at the Nuclear Envelope
While the function of dynein at the nuclear envelope may seem logical, as a factor linking centrosomes to the NE (discussed in ref. Citation32), it is less clear what the function of kinesin-1 at the nuclear envelope during mitotic entry may be. During G2/prophase centrosome separation is initiated, which in mammalian cells appears to depend mainly on the kinesin-5 motor Eg5.Citation20,Citation33–Citation35 During prophase centrosome separation, centrosomes remain closely associated with the nuclear envelope. Why then would a motor be activated at this time that actively pushes the centrosomes away from the nucleus? One possible explanation may be that, for centrosomes to migrate efficiently along the nucleus during separation, they need to be loosely coupled to the nuclear envelope. If they are pulled onto the nuclear envelope too tightly by the unbalanced action of dynein, a high amount of friction between centrosomes and nuclear envelope may be generated during centrosome migration, which will prevent efficient movement of centrosomes along the nuclear envelope. This implies that loss of kinesin-1 should slow down centrosome migration dynamics, a hypothesis that could be tested in the future. Similarly, the action of kinesin-1 might prevent mechanical damage to the nuclear envelope or centrosome due to unbalanced dynein activity pulling the centrosome into the nuclear envelope. Alternatively, it is now becoming clear that most cellular transport cargoes associate simultaneously with motors of opposite directionality,Citation1 even though many cargoes undergo mostly unidirectional movement, suggesting that only one of the two motors is active at any given time. However, it is possible that “unidirectional transport” in fact consists of predominant movement in one direction, but with short intermittent episodes of movement in the opposite direction (reviewed in ref. Citation1 and Citation36), for example when a blockage is encountered on a microtubule, and that the possibility for bidirectional movement improves the efficiency of cargo transport. Perhaps this type of biased, bidirectional transport is such a fundamental aspect of all transport that every cargo associates with motors of opposite polarity, even when one of the motors is very dominant over the other. In agreement with this view, both dynein and kinesin-1 were implicated in nuclear migration in such diverse processes as neuronal migration in mammalsCitation37,Citation38 and epithelial morphogenesis in worm embryos.Citation28 This idea is further supported by observations in cultured insect cells, which show that inhibition of a motor of one polarity can completely block organelle movement in both directions.Citation39
Summary and Future Directions
While microtubule motors clearly fulfill important functions at the nuclear envelope, we are still very far from understanding the molecular basis of the function of these motors function at the nuclear envelope. It is especially unclear why there are so many different ways in which motors can be recruited to the nuclear envelope.
It is tempting to speculate that different recruitment pathways of the motors are involved in different processes. For example, SUN-KASH domain containing proteins may be involved in linking dynein to the nuclear envelope during cell migration and meiotic chromosome pairing, while nuclear pore complexes may target dynein to the nucleus to facilitate the cell division-related functions of dynein. Direct comparison of the contribution of the different recruitment pathways to the different functions (i.e., using different experimental systems) may provide more information about their relative importance in these different processes.
Alternatively, multiple recruitment pathways may simply act together to promote all functions of dynein, for example to allow for very precise regulation of dynein recruitment or activation. In vitro reconstitution of protein complex formation of complicated interaction networks (like those between BICD2, Kinesin-1 and RanBP2) will hopefully yield a better understanding of how these protein complexes are built up and whether certain interactions are competitive or rather co-operative. Once the protein complex assembly is understood in more detail, the next challenge will be to determine how these protein complexes are regulated in space and time.
Similarly, much can still be learned about the different functions of motors at the nuclear envelope. As motors like kinesin-1 and dynein have many different localizations and functions in the cell, it is often difficult to ascribe an observed phenotype in a loss-of-function approach to a specific pool of the motor, like the nuclear envelope-associated pool. Therefore, new perturbations are needed that specifically deplete the motors from the nuclear envelope, as has been done with dynein at the kinetochore.Citation40 These will be challenging experiments, as it is often difficult to remove proteins from a specific localization without perturbing other processes as well.
Finally, it will be interesting to determine whether additional motors localize to the nuclear envelope that might also affect motor balances at the nuclear envelope. Systematic analysis of motor localization and function in nuclear positioning is now possible due to the development of RNAi libraries targeting the entire set of microtubule motorsCitation33,Citation41,Citation42 and recent developments in high throughput GFP-tagging of proteins.Citation43
Figures and Tables
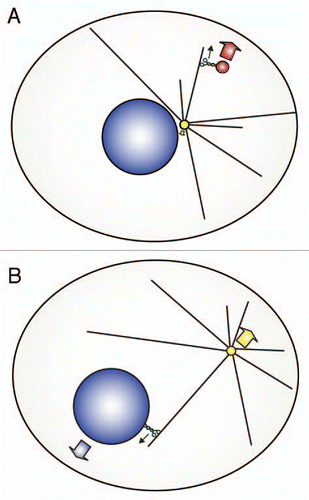
Figure 1 Movement of cargoes and the microtubule network during cargo transport. (A) Kinesin-1 associates with a vesicle and transports it towards the plus-end of a microtubule embedded in the centrosome. As the vesicle is small compared to the microtubule network, the vesicles is displaced relative to the microtubule network (large red arrow), while the microtubule network remains largely stationary (small yellow arrow). (B) Kinesin-1 associates with the nucleus and transports it towards the plus-end of a microtubule plus-end of a microtubule embedded in the centrosome. As the nucleus is very large, the nucleus and the microtubule network are displaced towards opposite sides (large blue and yellow arrows). In normal cells, dynein activity prevents separation of nucleus and centrosome, but in the absence of dynein activity (depicted here) kinesin-1 pushes centrosome and nucleus apart.
References
- Welte MA. Bidirectional transport along microtubules. Curr Biol 2004; 14:525 - 537
- Xiang X, Beckwith SM, Morris NR. Cytoplasmic dynein is involved in nuclear migration in Aspergillus nidulans. Proc Natl Acad Sci USA 1994; 91:2100 - 2104
- Gonczy P, Pichler S, Kirkham M, Hyman AA. Cytoplasmic dynein is required for distinct aspects of MTOC positioning, including centrosome separation, in the one cell stage Caenorhabditis elegans embryo. J Cell Biol 1999; 147:135 - 150
- Reinsch S, Karsenti E. Movement of nuclei along microtubules in Xenopus egg extracts. Curr Biol 1997; 7:211 - 214 DOI: S0960-9822(97)70092-7
- Robinson JT, Wojcik EJ, Sanders MA, McGrail M, Hays TS. Cytoplasmic dynein is required for the nuclear attachment and migration of centrosomes during mitosis in Drosophila. J Cell Biol 1999; 146:597 - 608
- Splinter D, et al. Bicaudal D2, dynein and kinesin-1 associate with nuclear pore complexes and regulate centrosome and nuclear positioning during mitotic entry. PLoS Biol 2010; 8:1000350; http://dx.doi.org/10.1371/journal.pbio.1000350
- Meyerzon M, Fridolfsson HN, Ly N, McNally FJ, Starr DA. UNC-83 is a nuclear-specific cargo adaptor for kinesin-1-mediated nuclear migration. Development 2009; 136:2725 - 2733
- Januschke J, Gervais L, Dass S, Kaltschmidt JA, Lopez-Schier H, St Johnston D, et al. Polar transport in the Drosophila oocyte requires Dynein and Kinesin I cooperation. Curr Biol 2002; 12:1971 - 1981 DOI: S0960982202013027
- Roux KJ, Crisp ML, Liu Q, Kim D, Kozlov S, Stewart CL, et al. Nesprin 4 is an outer nuclear membrane protein that can induce kinesin-mediated cell polarization. Proc Natl Acad Sci USA 2009; 106:2194 - 2199 DOI: 0808602106.0.1073/pnas.0808602106
- Xiang X, Fischer R. Nuclear migration and positioning in filamentous fungi. Fungal Genet Biol 2004; 41:411 - 419; http://dx.doi.org/10.1016/j.fgb.2003.11.010.S1087184503002081
- Moore JK, Cooper JA. Coordinating mitosis with cell polarity: Molecular motors at the cell cortex. Semin Cell Dev Biol 2010; 21:283 - 289 DOI: S1084-9521(10)00021-2.10.1016/j.semcdb.2010.01.020
- Starr DA. A nuclear-envelope bridge positions nuclei and moves chromosomes. J Cell Sci 2009; 122:577 - 586
- Tsai LH, Gleeson JG. Nucleokinesis in neuronal migration. Neuron 2005; 46:383 - 388 DOI: S0896-6273(05)00349-1.10.1016/j.neuron.2005.04.013
- Burke B, Roux KJ. Nuclei take a position: managing nuclear location. Dev Cell 2009; 17:587 - 597 DOI: S1534-5807(09)00440-7.10.1016/j.devcel.2009.10.018
- Starr DA. Communication between the cytoskeleton and the nuclear envelope to position the nucleus. Mol Biosyst 2007; 3:583 - 589; http://dx.doi.org/10.1039/b703878j
- Busson S, Dujardin D, Moreau A, Dompierre J, De Mey JR. Dynein and dynactin are localized to astral microtubules and at cortical sites in mitotic epithelial cells. Curr Biol 1998; 8:541 - 544
- Dujardin DL, Vallee RB. Dynein at the cortex. Curr Opin Cell Biol 2002; 14:44 - 49 DOI: S0955067401002927
- Malone CJ, Misner L, Le Bot N, Tsai MC, Campbell JM, Ahringer J, et al. The C. elegans hook protein, ZYG-12, mediates the essential attachment between the centrosome and nucleus. Cell 2003; 115:825 - 836 DOI: S0092867403009851
- Morris NR. Nuclear positioning: the means is at the ends. Curr Opin Cell Biol 2003; 15:54 - 59
- Tanenbaum ME, Macurek L, Galjart N, Medema RH. Dynein, Lis1 and CLIP-170 counteract Eg5-dependent centrosome separation during bipolar spindle assembly. EMBO J 2008; 27:3235 - 3245 DOI: emboj2008242.0.1038/emboj.2008.242
- Cai Y, Singh BB, Aslanukov A, Zhao H, Ferreira PA. The docking of kinesins, KIF5B and KIF5C, to Ranbinding protein 2 (RanBP2) is mediated via a novel RanBP2 domain. J Biol Chem 2001; 276:41594 - 41602
- Payne C, Rawe V, Ramalho-Santos J, Simerly C, Schatten G. Preferentially localized dynein and perinuclear dynactin associate with nuclear pore complex proteins to mediate genomic union during mammalian fertilization. J Cell Sci 2003; 116:4727 - 4738; http://dx.doi.org/10.1242/jcs.00784.116/23/4727
- Grigoriev I, Splinter D, Keijzer N, Wulf PS, Demmers J, Ohtsuka T, et al. Rab6 regulates transport and targeting of exocytotic carriers. Dev Cell 2007; 13:305 - 314
- Cho KI, Yi H, Desai R, Hand AR, Haas AL, Ferreira PA, et al. RANBP2 is an allosteric activator of the conventional kinesin-1 motor protein, KIF5B, in a minimal cell-free system. EMBO Rep 2009; 10:480 - 486
- Fridolfsson HN, Ly N, Meyerzon M, Starr DA. UNC-83 coordinates kinesin-1 and dynein activities at the nuclear envelope during nuclear migration. Dev Biol 2010; 338:237 - 250 DOI: S0012-1606(09)01393-1.10.1016/j.ydbio.2009.12.004
- Houalla T, Hien Vuong D, Ruan W, Suter B, Rao Y. The Ste20-like kinase misshapen functions together with Bicaudal-D and dynein in driving nuclear migration in the developing drosophila eye. Mech Dev 2005; 122:97 - 108 DOI: S0925-4773(04)00210-2.10.1016/j.mod.2004.08.005
- Swan A, Nguyen T, Suter B. Drosophila Lissencephaly-1 functions with Bic-D and dynein in oocyte determination and nuclear positioning. Nat Cell Biol 1999; 1:444 - 449; http://dx.doi.org/10.1038/15680
- Fridolfsson HN, Ly N, Meyerzon M, Starr DA. UNC-83 coordinates kinesin-1 and dynein activities at the nuclear envelope during nuclear migration. Dev Biol 338:237 - 250
- Bader JR, Vaughan KT. Dynein at the kinetochore: Timing, Interactions and Functions. Semin Cell Dev Biol 2010; 21:269 - 275 DOI: S1084-9521(09)00260-2.10.1016/j.semcdb.2009.12.015
- Salina D, Bodoor K, Eckley DM, Schroer TA, Rattner JB, Burke B, et al. Cytoplasmic dynein as a facilitator of nuclear envelope breakdown. Cell 2002; 108:97 - 107 DOI: S0092867401006286
- Sato A, Isaac B, Phillips CM, Rillo R, Carlton PM, Wynne DJ, et al. Cytoskeletal forces span the nuclear envelope to coordinate meiotic chromosome pairing and synapsis. Cell 2009; 139:907 - 919 DOI: S0092-8674(09)01365-8.10.1016/j.cell.2009.10.039
- Tanenbaum ME, Akhmanova A, Medema RH. Dynein at the nuclear envelope. EMBO Rep 2010; 11:649 DOI: embor2010127.10.1038/embor.2010.127
- Tanenbaum ME, Macrek L, Janssen A, Geers EF, Alvarez-Fernández M, Medema RH. Kif15 cooperates with eg5 to promote bipolar spindle assembly. Curr Biol 2009; 19:1703 - 1711 DOI: S0960-9822(09)01601-7.10.1016/j.cub.2009.08.027
- Whitehead CM, Rattner JB. Expanding the role of HsEg5 within the mitotic and post-mitotic phases of the cell cycle. J Cell Sci 1998; 111:2551 - 2561
- Woodcock SA, Rushton HJ, Castañeda-Saucedo E, Myant K, White GR, Blyth K, et al. Tiam1-Rac signaling counteracts Eg5 during bipolar spindle assembly to facilitate chromosome congression. Curr Biol 2010; 20:669 - 675 DOI: S0960-9822(10)00220-4.10.1016/j.cub.2010.02.033
- Holzbaur EL, Goldman YE. Coordination of molecular motors: from in vitro assays to intracellular dynamics. Curr Opin Cell Biol 2010; 22:4 - 13 DOI: S0955-0674(10)00002-5.10.1016/j.ceb.2009.12.014
- Zhang X, Lei K, Yuan X, Wu X, Zhuang Y, Xu T, et al. SUN1/2 and Syne/Nesprin-1/2 complexes connect centrosome to the nucleus during neurogenesis and neuronal migration in mice. Neuron 2009; 64:173 - 187 DOI: S0896-6273(09)00630-8.10.1016/j.neuron.2009.08.018
- Tanaka T, et al. Lis1 and doublecortin function with dynein to mediate coupling of the nucleus to the centrosome in neuronal migration. J Cell Biol 2004; 165:709 - 721 DOI: 10.1083/jcb.200309025.jcb.200309025
- Ally S, Larson AG, Barlan K, Rice SE, Gelfand VI. Opposite-polarity motors activate one another to trigger cargo transport in live cells. J Cell Biol 2009; 187:1071 - 1082 DOI: jcb.200908075.10.1083/jcb.200908075
- Yang Z, Tulu US, Wadsworth P, Rieder CL. Kinetochore dynein is required for chromosome motion and congression independent of the spindle checkpoint. Curr Biol 2007; 17:973 - 980
- Goshima G, Vale RD. The roles of microtubulebased motor proteins in mitosis: comprehensive RNAi analysis in the Drosophila S2 cell line. J Cell Biol 2003; 162:1003 - 1016
- Zhu C, Zhao J, Bibikova M, Leverson JD, Bossy-Wetzel E, Fan JB, et al. Functional analysis of human microtubule-based motor proteins, the kinesins and dyneins, in mitosis/cytokinesis using RNA interference. Mol Biol Cell 2005; 16:3187 - 3199
- Poser I, Sarov M, Hutchins JR, Hériché JK, Toyoda Y, Pozniakovsky A, et al. BAC TransgeneOmics: a high-throughput method for exploration of protein function in mammals. Nat Methods 2008; 5:409 - 415 DOI: nmeth.1199.10.1038/nmeth.1199