Abstract
Chemical synaptic transmission between neurons is believed to take place at specialized sites of cell contact, comprising presynaptic terminals and postsynaptic membranes. Neurotransmitter release has been shown to occur also extrasynaptically, mainly targeting glial cells. In a recent study, we investigated the mechanism of extrasynaptic glutamate and ATP release along sensory axons in the olfactory nerve layer. Transmitter release was mediated by calcium-dependent vesicle fusion and triggered calcium transients in adjacent glial cells. These calcium transients were coupled to vasoresponses, indicating that glial calcium signalling mediates neurovascular coupling not only in synaptic brain regions such as gray matter, but also in brain regions devoid of synapses.
Chemical synapses are complex structures enabling cell-to-cell communication in the nervous system. Neurotransmitters are stored in vesicles and are released upon depolarization of the presynaptic terminal into the synaptic cleft, where neurotransmitter molecules bind to receptors of the postsynaptic membrane. Neurotransmitters can also be released extrasynaptically, e.g., from the cell soma or along axons.Citation1–Citation3 Glial cells appear to be main targets of extrasynaptically released neurotransmitters, in particular of ATP.Citation4–Citation6 Only few studies, however, have analyzed the mechanism of ATP release from neurons. Recently, a vesicular nucleotide transporter has been cloned and charac- terized,Citation7 and quantal release of ATP from synaptic terminals has been measured by means of electrophysiological techniques,Citation8 suggesting vesicular ATP release. Whether this release mechanism is also employed by neurons to secrete ATP along axons has not been investigated before.
In our recent publication we used specialized glial cells in the olfactory nerve layer, olfactory ensheathing cells (OECs), to monitor neurotransmitter release from axons of olfactory receptor neurons.Citation9 OECs ensheath bundles of receptor axons and are equipped with various neurotransmitter receptors, rendering them ideal to detect ATP and other neurotransmitters released by axons. Action potential firing of receptor axons results in calcium signaling in OECs that is mediated by ATP as well as glutamate, indicating release of ATP and glutamate from olfactory receptor axons.Citation9 Neurotransmitter release from receptor axons is calcium-dependent, a pre-requisite for vesicle fusion. We could also directly demonstrate calcium-dependent vesicle fusion by using the vesicle fusion marker protein synapto-pHluorin expressed in olfactory receptor axons.Citation9 Vesicular release of ATP from synapses and cell somata was proposed earlier, mainly based on the kinetics of postsynaptic responses resembling quantal release.Citation1,Citation8 In these studies, however, the effect of suppressing vesicular release was not tested, nor were optional mechanisms ruled out to unequivocally demonstrate the exocytotic nature of the release process. In our study, preventing vesicle fusion by botulinum toxin A entirely suppressed ATP and glutamate release from olfatory receptor axons.Citation9 In addition, when neurotransmitter loading of vesicles was inhibited with bafilomycin A1, action potential firing failed to release ATP and glutamate. Blocking diffusion of neurotransmitters through pores such as gap junction hemi-channels and large-conductance P2X7 receptor channels with carbenoxelone, in contrast, had no effect.Citation9 Thus, in the nerve layer of the olfactory bulb, ATP and glutamate are released from receptor axons via exocytosis and trigger calcium rises in adjacent glial cells. Extrasynaptic neuron-glia communication mediated by ATP was also demonstrated between dorsal root ganglion (DRG) cell somata and satellite glial cells, where a quantal, vesicular release mechanism was proposed.Citation1 Interestingly, a recent publication demonstrated non-vesicular release of ATP from axons of DRG cells in culture that was insensitive to bafilomycin A1 and botulinum toxin.Citation10 In fact, action potential firing in DRG cells for 1 to 5 min at 10 Hz resulted in axon swelling, which led to opening of volume-activated anion channels, enabling diffusion of ATP out of the axons. The ATP concentration measured after DRG cell stimulation was rather low and ranged in a span between picomolar to low nanomolar concentrations.Citation10 The extracellular ATP concentration in the olfactory nerve following stimulation of receptor axons axons (20 Hz for 0.5 s), in contrast, was in the micromolar range, as estimated from the current response in sniffer cells that equals the currents measured upon puff application of 100 µM ATP.Citation9 Taken together, these studies show that more than one mechanism of extra-synaptic axonal ATP release exists, which may differ with respect to the cell type (type of neuron, culture versus tissue preparation), release capacity (low versus high amount of ATP) and stimulation duration (seconds versus minutes).
In synaptic regions, astrocytes respond to neurotransmitters with calcium transients which lead to the release of vasoactive substances from astrocytic endfeet, resulting in changes in the diameter of adjacent blood capillaries and hence blood flow.Citation11 Thereby, astrocytes link neuronal activity to changes in hemodynamics, a mechanism termed neurovascular coupling. However, roughly half of the brain consists of white matter, i.e., axon tracts in which synapses are sparse or even absent. Is neurovascular coupling present in these brain regions, and if yes, do white matter glial cells play a similar role in linking neuronal activity to hemodynamics as described for astrocytes in gray matter? To address these questions, we visualized blood vessels by injection of the fluorescent dye sulforhodamine 101 into blood vessels of the intact, isolated olfactory bulb and elicited calcium signaling in OECs. Glial calcium transients evoked by puff application of ATP or axonal stimulation were followed by a constriction of adjacent blood vessels.Citation9 Since blood vessels themselves may express purinoceptors,Citation12 it was unclear whether vasoconstriction was mediated by OEC calcium signaling or directly via purinergic effects in the blood vessel wall. Therefore, we elicited calcium transients by focal laser photolysis of “caged calcium” (NP-EGTA) in single NP-EGTA-loaded OECs.Citation9 This resulted in vasoconstriction restricted to a short segment of a blood vessel directly adjacent to the stimulated OEC, while blood vessels further apart were not affected, indicating that calcium signaling in OECs is sufficient to trigger local vasoresponses. Hence, glia-mediated neurovascular coupling is not restricted to gray matter astrocytes, where neuron-glia communication is mediated via synaptically released neurotransmitters, but also occurs in brain regions devoid of synapses. In the olfactory bulb, neurovascular coupling is mediated by OECs in the nerve layer and by astrocytes in deeper layers (). Astrocytic calcium signaling is triggered by glutamate released by olfactory receptor axon terminalsCitation13 and GABA released by periglomerular interneurons.Citation14 We could also show that olfactory receptor axon terminals release ATP evoking calcium transients in periglomerular astrocytes,Citation15 however, it is still unclear whether calcium signaling evoked by ATP in olfactory bulb astrocytes results in vasoresponses of blood vessels in the glomerular layer.
The studies of our group and others demonstrate a significant contribution of extrasynaptic neurotransmitter release to neuron-glia communication. Investigating the mechanisms and functional consequences of extrasynaptic neuron-glia interactions is an important step towards our understanding of brain function, but also brain pathology, since many neuronal disorders such as multiple sclerosis and vanishing white matter disease affect white matter.
Figures and Tables
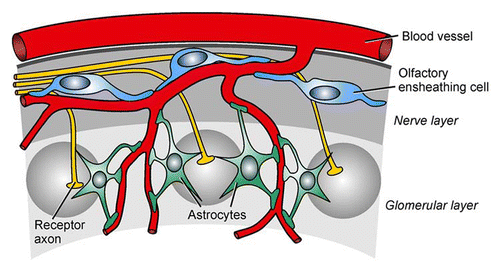
Figure 1 Glia-blood vessel relationship in the olfactory bulb. Olfactory ensheathing cells receive input from olfactory receptor axons via extrasynaptically released ATP and glutamate in the nerve layer, while astrocytes detect synaptically released neurotransmitters in the glomerular layer. Both types of glial cells link neuronal activity to responses of blood vessels via calcium signaling (see text for details).
Acknowledgements
Financial support by the Deutsche Forschungsgemeinschaft (LO 779/2 and LO 779/3) is gratefully acknowledged.
Addendum to:
References
- Zhang X, Chen Y, Wang C, Huang LY. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci USA 2007; 104:9864 - 9869
- Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat Neurosci 2007; 10:311 - 320
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci 2007; 10:321 - 330
- Hamilton N, Vayro S, Kirchhoff F, Verkhratsky A, Robbins J, Gorecki DC, Butt AM. Mechanisms of ATP- and glutamate-mediated calcium signalling in white matter astrocytes. Glia 2008; 56:734 - 749
- Stevens B, Fields RD. Response of Schwann cells to action potentials in development. Science 2000; 287:2267 - 2271
- Rieger A, Deitmer JW, Lohr C. Axon-glia communication evokes calcium signaling in olfactory ensheathing cells of the developing olfactory bulb. Glia 2007; 55:352 - 359
- Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, et al. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci USA 2008; 105:5683 - 5686
- Pankratov Y, Lalo U, Verkhratsky A, North RA. Vesicular release of ATP at central synapses. Pflügers Arch 2007; 452:589 - 597
- Thyssen A, Hirnet D, Wolburg H, Schmalzing G, Deitmer JW, Lohr C. Ectopic vesicular neurotransmitter release mediates neurovascular coupling via glial calcium signaling. Proc Natl Acad Sci USA 2010; 107:15258 - 15263
- Fields RD, Ni Y. Nonsynaptic communication through ATP release from volume-activated anion channels in axons. Sci Signal 2010; 3:73
- Carmignoto G, Gómez-Gonzalo M. The contribution of astrocyte signaling to neurovascular coupling. Brain Res Rev 2010; 63:138 - 148
- Burnstock G. Control of vascular tone by purines and pyrimidines. Br J Pharmacol 2010; 161:527 - 529
- Petzold GC, Albeanu DF, Sato TF, Murthy VN. Coupling of neural activity to blood flow in olfactory glomeruli is mediated by astrocytic pathways. Neuron 2008; 58:897 - 910
- Doengi M, Hirnet D, Coulon P, Pape HC, Deitmer JW, Lohr C. GABA uptake-dependent Ca2+ signaling in developing olfactory bulb astrocytes. Proc Natl Acad Sci USA 2009; 106:17570 - 17575
- Doengi M, Deitmer JW, Lohr C. New evidence for purinergic signaling in the olfactory bulb: A2A and P2Y1 receptors mediate intracellular calcium release in astrocytes. FASEB J 2008; 22:2368 - 2378