Abstract
MELK has been implicated in a large variety of functions. Because its level is elevated in cancer tissues and it is involved in cell proliferation, MELK is considered as a potential therapeutic target for cancers. In a recent, study we have shown that MELK is involved in cytokinesis in early Xenopus laevis embryos. MELK dynamically accumulates at the cell cortex including a narrow band corresponding to the presumptive division furrow shortly before cytokinesis onset. MELK co-localizes and interacts with anillin an important regulator of cytokinesis. In addition, MELK overexpression interferes with accumulation at the cleavage furrow of activated Rho GTPase another crucial regulator of cytokinesis. Interestingly, our study also revealed that a transition implying a change in the direction of asymmetric furrow ingression occurs during early development. After this transition, MELK, as well as other proteins involved in cytokinesis, do not localize anymore as a band at the equatorial cortex but still localizes at the cell cortex. Our results indicate that cortical localization is an important feature of MELK in X. laevis embryos.
MELK is a serine/threonine protein kinase originally identified in Xenopus oocytes and embryos as Eg3,Citation1 and later in the mouseCitation2 due to its specific expression pattern in early embryos. MELK was shown to be involved in the control of cell proliferation,Citation3,Citation4 regulation of apoptosis,Citation5 inhibition of spliceosome assembly,Citation6 haematopoiesis,Citation7 asymmetric cell divisionsCitation8 and cell cycle.Citation9,Citation10 Although MELK has been implicated in such a large variety of biological processes, its precise function remains elusive. In cancer tissues, it was shown that MELK level is dramatically increased and could be beneficial to tumoral cells.Citation11 In agreement with this view, MELK activity was found to inhibit apoptosis in breast cancer cells.Citation5 This lead to the proposal that MELK could be a therapeutic target for several types of cancer.Citation4,Citation5,Citation11,Citation12
MELK expression is precisely controlled during the cell cycle in two ways. Firstly, the kinase is expressed in cells engaged into the cell cycle, but its amount decreases to undetectable levels in cells that have left the cell cycle to differentiate.Citation13 Secondly, in cycling cells, MELK mRNA and protein levels fluctuate during the cell cycle with a moderate increase during mitosis.Citation11,Citation13,Citation14 Interestingly, a correlation was established between high levels of MELK and the malignancy grade in brain tumors.Citation12,Citation15 Moreover, high levels of MELK were also associated with poor prognosis in breast cancer.Citation16 Thus MELK appears also to be an important prognosis marker for some cancers.
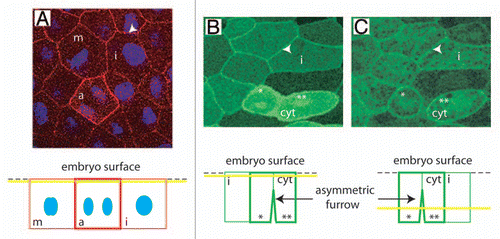
To extend our understanding of the role of MELK, we studied it in Xenopus laevis oocytes and embryos where MELK expression, phosphorylation and activity are tightly controlled.Citation1,Citation17,Citation18 In our recent study, we discovered that MELK regulates cytokinesis in early embryos.Citation19 Cytokinesis is the process by which a mother cell divides into two topologically distinct daughter cells. Cell membrane ingression is mediated by an actomyosin-based contractile ring, the assembly and constriction of which are orchestrated by the small GTPase RhoA. Both knockdown and overexpression of Xenopus MELK lead to abortive divisions in embryos indicating that MELK must be finely-tuned for cytokinesis to be completed. In agreement with a role in cytokinesis, shortly before its onset the endogenous MELK accumulates as a band at the equatorial cortex which ultimately ingresses and forms the cytokinetic furrow. This band appears very similar to the previously described localization of active RhoA in Xenopus early embryos.Citation20 A dynamic re-localization of MELK occurs at the cell periphery and equatorial cortex shortly before cytokinesis. Using a FRET-based probe, we found that MELK cortical localization correlates with xMELK conformational rearrangement. Furthermore, MELK overexpression leads to concurrent cytokinesis failure and impaired accumulation of active Rho at the division site, which could explain abortive cytokinesis. Interestingly, xMELK co-localizes with anillin, a crucial cytokinetic protein at the equatorial cortex. Moreover, the two proteins can be coimmunoprecipitated. At present the role of this association is unknown. However because anillin interacts with several molecules controlling cytokinesis, a plausible hypothesis is that MELK phosphorylates an anillin-interacting partner. Unexpectedly, the localization of MELK at the equatorial cortex as well as other cytokinetic proteins like actin, myosin heavy chain and active Rho but not anillin is regulated during development. Indeed, MELK does not accumulate at the equatorial cortex in gastrula embryos. This change appears to be correlated with the cell size considerably decreasing during embryo cleavage. In addition, an inversion of asymmetric furrowing occurs during this period. This leads to asymmetric furrow progression from basal to apical cortex in embryo epithelial cells that have passed the transition in the cytokinesis mode (). The role of asymmetric furrowing is unknown, but it is encountered in divers organism including worm and ascidian embryosCitation21–Citation23 as well as MDCKII cells cultured in vitro.Citation24 In the latter case, the asymmetric furrowing proceeds from the basolateral side towards the apical domain. This indicates that asymmetric furrowing is not only specific to embryo and was conserved through evolution. This in turn may suggest that it could have an important function remaining, however, to be elucidated. After the transition in the cytokinesis mode, MELK accumulates at the cell cortex during the metaphase to anaphase transition (), similarly as in human cells.Citation25 Thus, MELK cortical localization is correlated with the metaphase to anaphase transition, which supposes that it may be related to spindle assembly checkpoint. Interestingly, a substantial amount of endogenous MELK is localized at the cell cortex also in interphase cells and mitotic cells until metaphase (arrowhead in ). Similarly, GFP-MELK fusion protein localizes at the cell cortex during interphase (arrowheads in and C). Therefore, when considering the cortical localization, two MELK pools can be distinguished: an interphasic MELK (iMELK) and a mitotic MELK (mMELK). This suggests that in cycling cells MELK could also have an important role during interphase. During mitosis, mMELK, by increasing the global MELK amount at the cortex may reinforce the action of iMELK, if iMELK and mMELK have the same substrate(s). However, mMELK and iMELK may phosphorylate different protein(s) and thus may have distinct functions during the cell cycle. Undoubtedly, it will be critical to identify MELK substrate(s) to understand its role. How the cortical localizations of iMELK and mMELK are regulated is linked to another intriguing question of how cells can manage the two MELK populations. It seems likely that phosphorylation of MELK, which appears complex because of the large number of identified sites phosphorylated in vivoCitation18,Citation26,Citation27 may have a role in such mechanism. When cells exit mitosis, the MELK level localized at the cortex returns to the interphase level. Interestingly, an abrupt degradation of approximately 50% of MELK in mitotic cells occurs specifically upon mitotic exit.Citation13 It will be interesting to determine if upon mitotic exit mMELK is targeted to degradation whereas iMELK level remains stable. The understanding how MELK is regulated, will contribute to define MELK function during the cell cycle. This knowledge may be valuable for future studies on the use of MELK as a therapeutic target.
Figures and Tables
Figure 1 MELK localization in epithelial cells of Xenopus gastrula embryos. (A) Indirect immunofluorescence with anti-MELK antibodies. Endogenous MELK is detected at the cell cortex in interphase cell (arrowhead) and further accumulates at the cell cortex at the metaphase to anaphase transition (m: metaphase, a: anaphase). The yellow line on the scheme at the bottom indicates the position of the confocal plane relative to the cell surface. DNA (blue) which is situated more profoundly in the cells was merged with overlaying MELK signal. (B and C) The mRN A coding for GFP tagged MELK inactive mutant (GFP-MELK K/R) which does not induce cytokinesis failure was microinjected in an embryo at the two cell stage and GFP-MELK K/R was followed in a live gastrula embryo. Two confocal planes taken at the same time are shown. GFP-MELK K/R is localized at the cell cortex of interphase cells (i: interphase cell, arrowhead in b and c) and further accumulates at the cell cortex in cytokinetic cell (cyt). Note that the apical domain of the cytokinetic cell is beginning its division whereas its baso-lateral membrane is already divided. * and ** indicate the two forming daughter cells to facilitate analysis of the original figure and the related scheme at the bottom.
Acknowledgments
Author thanks Jacek Kubiak for critical reading of this manuscript. This work was supported by the C.N.R.S., the Ligue Départementale contre le Cancer (22 et 35) and the ARC.
Addendum to:
References
- Paris J, Philippe M. Poly(A) metabolism and polysomal recruitment of maternal mRNAs during early Xenopus development. Dev Biol 1990; 140:221 - 224
- Heyer BS, Warsowe J, Solter D, Knowles BB, Ackerman SL. New member of the Snf1/AMPK kinase family Melk, is expressed in the mouse egg and preimplantation embryo. Mol Reprod Dev 1997; 47:148 - 156
- Nakano I, Paucar AA, Bajpai R, Dougherty JD, Zewail A, Kelly TK, et al. Maternal embryonic leucine zipper kinase (MELK) regulates multipotent neural progenitor proliferation. J Cell Biol 2005; 170:413 - 427
- Hebbard LW, Maurer J, Miller A, Lesperance J, Hassell J, Oshima RG, Terskikh AV. Maternal embryonic leucine zipper kinase is upregulated and required in mammary tumor-initiating cells in vivo. Cancer Res 2010; 70:8863 - 8873
- Lin ML, Park JH, Nishidate T, Nakamura Y, Katagiri T. Involvement of maternal embryonic leucine zipper kinase (MELK) in mammary carcinogenesis through interaction with Bcl-G, a pro-apoptotic member of the Bcl-2 family. Breast Cancer Res 2007; 9:17
- Vulsteke V, Beullens M, Boudrez A, Keppens S, Van Eynde A, Rider MH, et al. Inhibition of spliceosome assembly by the cell cycle-regulated protein kinase MELK and involvement of splicing factor NIPP1. J Biol Chem 2004; 279:8642 - 8647
- Saito R, Tabata Y, Muto A, Arai K, Watanabe S. Melk-like kinase plays a role in hematopoiesis in the zebra fish. Mol Cell Biol 2005; 25:6682 - 6693
- Cordes S, Frank CA, Garriga G. The C. elegans MELK ortholog PIG-1 regulates cell size asymmetry and daughter cell fate in asymmetric neuroblast divisions. Development 2006; 133:2747 - 2756
- Davezac N, Baldin V, Blot J, Ducommun B, Tassan JP. Human pEg3 kinase associates with and phosphorylates CDC25B phosphatase: a potential role for pEg3 in cell cycle regulation. Oncogene 2002; 21:7630 - 7641
- Mirey G, Chartrain I, Froment C, Quaranta M, Bouche JP, Monsarrat B, et al. CDC25B phosphorylated by pEg3 localizes to the centrosome and the spindle poles at mitosis. Cell Cycle 2005; 4:806 - 811
- Gray D, Jubb AM, Hogue D, Dowd P, Kljavin N, Yi S, et al. Maternal embryonic leucine zipper kinase/murine protein serine-threonine kinase 38 is a promising therapeutic target for multiple cancers. Cancer Res 2005; 65:9751 - 9761
- Nakano I, Masterman-Smith M, Saigusa K, Paucar AA, Horvath S, Shoemaker L, et al. Maternal embryonic leucine zipper kinase is a key regulator of the proliferation of malignant brain tumors, including brain tumor stem cells. J Neurosci Res 2008; 86:48 - 60
- Badouel C, Chartrain I, Blot J, Tassan JP. Maternal embryonic leucine zipper kinase is stabilized in mitosis by phosphorylation and is partially degraded upon mitotic exit. Exp Cell Res 2010; 316:2166 - 2173
- Whitfield ML, Sherlock G, Saldanha AJ, Murray JI, Ball CA, Alexander KE, et al. Identification of genes periodically expressed in the human cell cycle and their expression in tumors. Mol Biol Cell 2002; 13:1977 - 2000
- Marie SK, Okamoto OK, Uno M, Hasegawa AP, Oba-Shinjo SM, Cohen T, et al. Maternal embryonic leucine zipper kinase transcript abundance correlates with malignancy grade in human astrocytomas. Int J Cancer 2008; 122:807 - 815
- Pickard MR, Green AR, Ellis IO, Caldas C, Hedge VL, Mourtada-Maarabouni M, Williams GT. Dysregulated expression of Fau and MELK is associated with poor prognosis in breast cancer. Breast Cancer Res 2009; 11:60
- Blot J, Chartrain I, Roghi C, Philippe M, Tassan JP. Cell Cycle Regulation of pEg3, a New Xenopus Protein Kinase of the KIN1/PAR-1/MARK Family. Dev Biol 2002; 241:327 - 338
- Badouel C, Korner R, Frank-Vaillant M, Couturier A, Nigg EA, Tassan JP. M-phase MELK Activity is Regulated by MPF and MAPK. Cell Cycle 2006; 5:883 - 889
- Le Page Y, Chartrain I, Badouel C, Tassan JP. A functional analysis of MELK in cell division reveals a transition in the mode of cytokinesis during Xenopus development. J Cell Sci 2011; 124:958 - 968
- Bement WM, Benink HA, von Dassow G. A microtubule-dependent zone of active RhoA during cleavage plane specification. J Cell Biol 2005; 170:91 - 101
- Audhya A, Hyndman F, McLeod IX, Maddox AS, Yates JR 3rd, Desai A, Oegema K. A complex containing the Sm protein CAR-1 and the RNA helicase CGH-1 is required for embryonic cytokinesis in Caenorhabditis elegans. J Cell Biol 2005; 171:267 - 279
- Maddox AS, Lewellyn L, Desai A, Oegema K. Anillin and the septins promote asymmetric ingression of the cytokinetic furrow. Dev Cell 2007; 12:827 - 835
- Prodon F, Chenevert J, Hebras C, Dumollard R, Faure E, Gonzalez-Garcia J, et al. Dual mechanism controls asymmetric spindle position in ascidian germ cell precursors. Development 2010; 137:2011 - 2021
- Reinsch S, Karsenti E. Orientation of spindle axis and distribution of plasma membrane proteins during cell division in polarized MDCKII cells. J Cell Biol 1994; 126:1509 - 1526
- Chartrain I, Couturier A, Tassan JP. Cell cycle dependent cortical localization of pEg3 protein kinase in Xenopus and human cells. Biol Cell 2006; 98:253 - 263
- Daub H, Olsen JV, Bairlein M, Gnad F, Oppermann FS, Korner R, et al. Kinase-selective enrichment enables quantitative phosphoproteomics of the kinome across the cell cycle. Mol Cell 2008; 31:438 - 448
- Dephoure N, Zhou C, Villen J, Beausoleil SA, Bakalarski CE, Elledge SJ, Gygi SP. A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci USA 2008; 105:10762 - 10767