Abstract
Chlamydia trachomatis is the causing agent of the most frequent bacterial sexually-transmitted diseases worldwide and is an underlying cause of chronic pelvic inflammatory diseases and cervical cancer. It is an obligate intracellular bacterium that establishes a close relationship with the Golgi complex and parasites the biosynthetic machinery of host cells. In a recent study, we have demonstrated that Rab14, a newly-described Golgi-associated Rab, is involved in the delivery of sphingolipids to the growing bacteria-containing vacuole. The interference with Rab14-controlled trafficking pathways delays chlamydial inclusion enlargement, decreases bacterial lipid uptake, negatively impact on bacterial differentiation, and reduces bacterial progeny and infectivity. C. trachomatis manipulation of host trafficking pathways for the acquisition of endogenously-biosynthesized nutrients arises as one of the characteristics of this highly evolved pathogen. The development of therapeutic strategies targeted to interfere with bacterium-host cell interaction is a new challenge for pharmacological approaches to control chlamydial infections.
C. trachomatis is a strictly human pathogen, with a tropism for the genital and ocular epithelia. C. trachomatis causes trachoma, an ocular infection that leads to blindness and sexually-transmitted diseases ranging from urethritis, cervicitis and epididimitis to pelvic inflammatory disease, ectopic pregnancy, tubal obstruction and infertility.Citation1 Persistent infections are lately linked to severe chronic inflammatory diseases and cancer.Citation2
As an obligate intracellular bacterium, Chlamydia trachomatis has evolved several strategies to enter the host cells and to avoid intracellular degradation through the generation of a safe and convenient membrane-bound vacuole (the inclusion) where it survives, growths and replicates.Citation3,Citation4 Chlamydia depends on host cells for key functions, particularly for nutrient acquisition.Citation5,Citation6 A family of bacterial integral inclusion membrane proteins (Incs), mostly of unknown function up to date, might promote interaction with host proteins involved in the delivery of nutrient-containing vesicles to the inclusion as well as in inclusion remodeling.Citation7,Citation8 Although, such processes are essential for the bacterium's intracellular survival, the exact nature of the molecular mechanisms that participate is little understood. Recent data indicating that an increasing number of intracellular pathogens manipulate Rab GTPases to the biogenesis of an amenable vacuole for survival lead to the speculation that Chlamydia subverts host vesicular trafficking by recruiting specific host Rab proteins.Citation9 Rab GTPases are key controllers of membrane trafficking and organelle identity by means of their involvement in the generation, transport, recognition and fusion of intracellular vesicles.Citation10 Several Rab GTPases were found to associate with the inclusion of various chlamydial species.Citation11 The role of each Rab may differ depending on its specific intracellular localization, as well as on the timing of recruitment to the inclusion. Since Chlamydia establishes a close relationship with the Golgi complex, we sought to determine whether Chlamydia exploits host vesicular trafficking machinery through the selective recruitment of a newly-described Golgi-associated Rab GTPase i.e., Rab14.Citation12,Citation13
Our finding of endogenous Rab14 surrounding chlamydial inclusions is significant since it constitutes the first report showing an endogenous Rab associated to the inclusions. The recruitment of Rab14 is observed once the inclusion reaches the perinucleus, approximately 10 h post infection (p.i.), and peaks at mid-term inclusions. At later stages of development, Rab14 is found associated to intra-inclusion vesicle-like structures. The unexpected finding of Rab14-enriched structures within the inclusion raises an unsolved question about the mechanisms by which organelles and membranes are translocated inside inclusions. Our results show that Rab14 recruitment is a bacteria-driven process, Brefeldin A-insensitive and independent of microtubules integrity ().
Multiple host trafficking pathways and intracellular lipid sources (Golgi-derived vesicles, multivesicular bodies and lipid droplets) are subverted by the bacterium to provide redundancy in the strategies developed for nutrient acquisition.Citation14–Citation16 The interception of vesicles full of endogenous, newly-synthesized lipids in transit from the Golgi complex to the plasma membrane is essential for bacterial growth and production of infectious progeny.Citation17,Citation18 The bacterial hijacking of Rab6 and Rab11 ensures the selective redirection of Golgi-derived vesicles to the inclusion.Citation19 Our results show that Chlamydia trachomatis promotes an additional overlapping mechanism for Golgi-derived lipids rerouting to the inclusion, through the recruitment of Rab14-enriched vesicles.Citation20
The detrimental impact on bacteria infectivity observed after disruption of Rab14-controlled transport events supports the idea that bacteria development and replication depend upon lipids delivery to the inclusion and are favored by the presence of a functional Rab14 at the chlamydial inclusion membrane. Overexpression of Rab14 negative mutants or endogenous Rab14 silencing disrupt sphingolipids transport assessed by confocal microscopy, and result in smaller chlamydial inclusions which failed to incorporate lipids, therefore having severe consequences on bacterial developmental cycle. Chlamydia cycles between two developmental forms: the infectious metabolically inactive elementary bodies (EBs) and the larger replicative bodies (RBs) that differ in their appearance in transmission electron microscopy (TEM). Rab14wt expressing cells reveal a normal Chlamydia developmental cycle, whereas less and abnormal bacterial forms (including bacterial ghosts and aberrant persistent bodies) are found in Rab14 negative mutants overexpressing cells coincidently with less sphingolipids capture by chlamydial organisms quantified by thin layer chromatography and spectrofluorometry. Rab14 negative mutants overexpression or endogenous Rab14 silencing dramatically impair the overall bacterial ability to replicate, as assessed by counting bacteria inside inclusions by TEM at 24 h p.i. and quantified by Inclusion Forming Units (IFUs) at 48 h p.i. Even though more than one Rab-controlled pathway is subverted by this bacterium, the detrimental effects of Rab14 silencing or Rab14 negative mutants overexpression reveal that this Rab plays a pivotal role in the delivery of newly-synthesized sphingolipids to the inclusion. The scheme summarizes the current knowledge of nutrient sources and lipid delivery trafficking pathways subvert by Chlamydia trachomatis ().
We have evidence that Chlamydia trachomatis induces the expression of host Rab14 (unpublished results). This consistent finding correlates with the requirement of host sphingolipids for bacterial growth and replication. The fact that the bacteria promote host Rab14 synthesis and consequently increase host cells lipid uptake, could constitute a reinforcing mechanism to ensure bacterial nutrition and survival. Understanding the molecular machinery involved in bacterium-host cell interaction might provide key means for the development of novel anti-chlamydial compounds that improve the outcomes observed with current antibiotic treatment.
Figures and Tables
Figure 1 Rab14 recruitment to chlamydial inclusions is a bacteria-driven process, independent of microtubules and Golgi integrity. HeLa cells infected with C. trachomatis serovar L2 (MOI 10) were fixed at 10 h p.i. Endogenous Rab14 was detected with rabbit polyclonal anti-Rab14 (1:100) followed by FITC-labeled goat anti-rabbit IgG (1:200) (green) in (A, C and D). Eukaryotic and bacterial DNA was evidenced by Hoescht staining (blue). (B) GFP-Rab14wt overexpressing cells (green) infected with C. trachomatis L2 (MOI 10) were incubated with 200 µg/ml Chloramphenicol (20 h) to inhibit bacterial protein synthesis. Cells were fixed at 24 h p.i. (C) Infected cells were treated with 1 µg/ml Brefeldin a (6 h) to disrupt Golgi apparatus. Mouse monoclonal anti-TGN 46 antibodies (1:200) followed by donkey anti-mouse Cy5-conjugated IgG (1:700) (red) were used to stain Golgi apparatus. (D) Infected cells were incubated with 20 µM Nocodazole (6 h) to depolymerize microtubules. Mouse anti-β-tubulin antibodies (1:200) followed by donkey anti-mouse Cy5-conjugated IgG (1:700) (red) were used to label microtubules. Insets show a magnification of the selected area of the cell. Bar, 10 µm. Endogenous Rab14 was clearly recruited to the inclusion as shown by rim-like green fluorescence staining around the entire periphery of the inclusion (A, C and D); whereas no recruitment was observed in Chloramphenicol-treated cells (B).
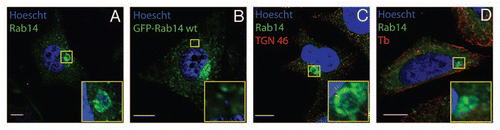
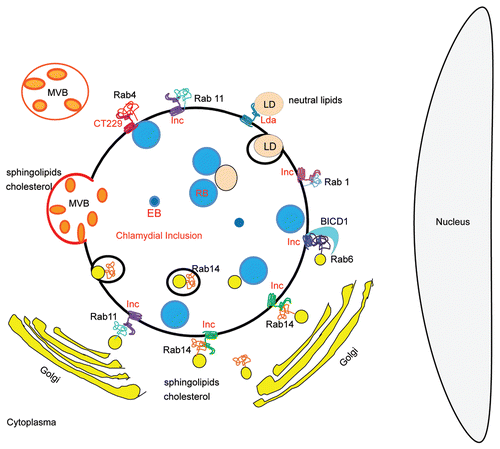
Figure 2 Schematic representation of lipid sources and Rab-controlled trafficking pathways usurp by Chlamydia trachomatis. RBs, Reticulate bodies are the replicative bacterial forms; EBs, Elementary bodies are the infective bacterial forms; Incs, Bacterial transmembrane proteins exposed at the inclusion membrane. Unidentified Incs are putative interacting factors with host proteins. CT229 is the Inc., from C. trachomatis that recruits Rab4; Rabs, Monomeric GTPases from host cells that control intracellular trafficking. Rab1, Rab4, Rab6, Rab11 and Rab14 have been found associated to C. trachomatis inclusion. BICD1 is a host Rab-6 interacting protein that is recruited to the chlamydial inclusion; MVBs, host multivesicular bodies, source of sphingolipids and cholesterol, are redirected to the chlamydial inclusion; LDs, Lipid droplets, host neutral lipids storage, are rerouted and translocated into the inclusion with the participation of chlamydial lipid droplet-associated proteins (Lda); Golgi-derived vesicles containing host endogenously-biosynthesized sphingolipids and cholesterol are targeted to the chlamydial inclusion. Note Golgi mini-stacks surrounding the inclusion. Chlamydia trachomatis intercepts Rab6-, Rab11- and Rab14-positive vesicles full of lipids, in transit from the Golgi apparatus to the plasma membrane. Rab14-labeled structures have been observed within inclusions at late-stages of chlamydial development.
Acknowledgments
We thank L. Mayorga for critically reading the manuscript. This work was supported by grants from the Mincyt (PICT 38077), CONICET, Sepcyt and Fundación Bunge y Born to M.T.D.
Addendum to:
References
- Carey AJ, Beagley KW. Chlamydia trachomatis, a hidden epidemic: effects on female reproduction and options for treatment. Am J Reprod Immunol 2010; 63:576 - 586
- Knowlton AE, Brown HM, Richards TS, Andreolas LA, Patel RK, Grieshaber SS. Chlamydia trachomatis infection causes mitotic spindle pole defects independently from its effects on centrosome amplification. Traffic 2011; 12:854 - 866; http://dx.doi.org/10.1111/j.1600-0854.2011.01204.x
- Fields KA, Hackstadt T. The Chlamydial inclusion: escape from the endocytic pathway. Annu Rev Cell Dev Biol 2002; 18:221 - 245
- Scidmore MA. Recent advances in Chlamydia subversion of host cytoskeletal and membrane trafficking pathways. Microbes Infect 2011; 13:527 - 535
- Cocchiaro JL, Valdivia RH. New insights into Chlamydia intracellular survival mechanisms. Cell Microbiol 2009; 11:1571 - 1578
- Robertson DK, Gu L, Rowe RK, Beatty WL. Inclusion biogenesis and reactivation of persistent Chlamydia trachomatis requires host cell sphingolipid biosynthesis. PLoS Pathog 2009; 5:1000664
- Dehoux P, Flores R, Dauga C, Zhong G, Subtil A. Multi-genome identification and characterization of Chlamydiae-specific type III secretion substrates: the Inc, proteins BMC Genomics 2011; 12:109
- Delevoye C, Nilges M, Dehoux P, Paumet F, Perrinet S, Dautry-Varsat A, et al. SNARE protein mimicry by an intracellular bacterium. PLoS Pathog 2008; 4:1000022
- Brumell JH, Scidmore MA. Manipulation of rab GTPase function by intracellular bacterial pathogens. Microbiol Mol Biol Rev 2007; 71:636 - 652
- Segev N. Coordination of intracellular transport steps by GTPases. Semin Cell Dev Biol 2011; 22:33 - 38
- Rzomp KA, Scholtes LD, Briggs BJ, Whittaker GR, Scidmore MA. Rab GTPases are recruited to chlamydial inclusions in both a species-dependent and species-independent manner. Infect Immun 2003; 71:5855 - 5870
- Kitt KN, Hernández-Deviez D, Ballantyne SD, Spiliotis ET, Casanova JE, Wilson JM. Rab14 regulates apical targeting in polarized epithelial cells. Traffic 2008; 9:1218 - 1231
- Junutula JR, De Maziére AM, Pede AA, Ervin KE, Advani RJ, van Dijk SM, et al. Rab14 is involved in membrane trafficking between the Golgi complex and endosomes. Mol Biol Cell 2004; 15:2218 - 2229
- Saka HA, Valdivia RH. Acquisition of nutrients by Chlamydiae: unique challenges of living in an intracellular compartment. Curr Opin Microbiol 2010; 13:4 - 10
- Beatty WL. Late endocytic multivesicular bodies intersect the Chlamydial inclusion in the absence of CD63. Infect Immun 2008; 76:2872 - 2881
- Cocchiaro JL, Kumar Y, Fischer ER, Hackstadt T, Valdivia RH. Cytoplasmic lipid droplets are trans-located into the lumen of the Chlamydia trachomatis parasitophorous vacuole. Proc Natl Acad Sci USA 2008; 105:9379 - 9384
- Moore ER, Fischer ER, Mead DJ, Hackstadt T. The chlamydial inclusion preferentially intercepts basolaterally directed sphingomyelin-containing exocytic vacuoles. Traffic 2008; 9:2130 - 2140
- Moore ER, Mead DJ, Dooley CA, Sager J, Hackstadt T. The trans-Golgi SNARE syntaxin 6 is recruited to the chlamydial inclusion membrane. Microbiology 2011; 157:830 - 838
- Rejman Lipinski A, Heymann J, Meissner C, Karlas A, Brinkmann V, Meyer TF, et al. Rab6 and Rab11 regulate Chlamydia trachomatis development and golgin-84-dependent Golgi fragmentation. PLoS Pathog 2009; 5:1000615
- Capmany A, Damiani MT. Chlamydia trachomatis intercepts Golgi-derived sphingolipids through a Rab14-mediated transport required for bacterial development and replication. PLoS One 2010; 5:14084