 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The production, maintenance and transmission of chemical signals often entail costs. Costs can arise, for instance, if signal production depends on the availability of limited nutritional resources or if signal transmission leads to attraction of predators. Many species effectively reduce these costs by signaling at specific times or in certain contexts only. We previously reported that breeding burying beetle females facultatively adjust their pheromone emission in response to their social environment, emitting high amounts of their chemical signal in the presence of a male partner, but not when providing uniparental care. Here we present data showing that chemical signaling is costly, and that higher investments in signaling result in reduced clutch sizes, but not a shorter life span, in the burying beetle Nicrophorus vespilloides.
The study of animal communication, a central focus of behavioral ecology, almost always involves some consideration of signal costs. This is largely because it is widely held that the maintenance of honest communication requires that signaling must incur ‘strategic’ costs.Citation1 Although Zahavi’s handicap principle is still the most widely accepted explanation for signal reliability,Citation2-Citation5 we have since learned that strategic costs are not the only force promoting signal evolution and maintenance, but rather, that there are other mechanisms that can enforce the honesty of a signal.Citation1,Citation6,Citation7 One example is indices, signals whose intensity is causally related to the quality being signaled, and which, due to physical or chemical constraints, cannot be faked.Citation1 However, even when a signal does not involve a strategic cost, it is not necessarily cost free to the signaler. Each signal will incur ‘efficacy’ costs, costs that the signaler has to pay to efficiently transmit the signal to the receiver.Citation1,Citation8 Efficacy costs are likely to be affected by background noise, as signal intensity must be increased the more the environment becomes visually, acoustically or chemically cluttered.
Although the costs of chemical signaling are less well studied than those of other signaling modalities, and the distinction between strategic and efficacy costs has rarely been made for chemical signals, studies have found, not surprisingly, that chemical signals also incur costs.Citation9-Citation13 Indirect evidence for costs of pheromone or allomone emission has already accumulated over a period of many years. Signalers in a wide array of species do not emit their chemical signals continuously, but rather, modulate their emission depending on the time,Citation14-Citation16 locationCitation17 or social environment.Citation18,Citation19 Such adjustment of signal quantity to communication context not only may improve the efficiency of information transfer due to better synchronization of sender and receiver, but likely reduce the physiological or ecological (i.e., attraction of parasites or predators) costs of signal production or transmission.Citation20,Citation21 One example comes from a study of the fruit fly, Drosophila grimshawi, where males modulate pheromone production depending on the presence of a rival male.Citation10 Males for whom pheromone deposition was artificially increased due to prolonged exposure to rival males suffered from a shorter life span than solitary males.
In a recent study, we found that female burying beetles Nicrophorus vespilloides are able to adjust their pheromone emission in relation to their social environment.Citation22 Burying beetles are well known for their elaborate parental care.Citation23 During the period of parental care, female beetles communicate their breeding state via a chemical signal, methyl geranate,Citation24 but they only do so when breeding with a male partner (i.e., in the present of a receiver), but not when breeding alone.Citation22 In the case of biparental care, the signal is important for the success of the breeding attempt because it helps the male to distinguish between his female partner and a non-breeding female intruder.Citation24,Citation25 Any intruder, if not repelled, would almost certainly kill the entire brood.Citation26
Pheromone modulation allows burying beetle females to economize on signal costs, but the costs of methyl geranate production or transmission remain unclear. Ecological costs (i.e., the attraction of predators or parasitoidsCitation20) are unlikely because the beetles are below ground in a crypt during breeding and signal emission. It is more likely that signal production results in metabolic costs. In addition, because the molecular structure of the chemical substance suggests that methyl geranate is an index,Citation24 the type of costs involved are likely efficacy costs, not strategic costs. In the present study, we evaluated the costs of chemical signaling by comparing fitness-relevant parameters (specifically, clutch size and longevity) of females breeding with a male partner (i.e., females that emit a high amount of methyl geranate) and solitary breeding females (i.e., females that emit a marginal amount of methyl geranate). Pairs of beetles (n = 15) and single females (n = 15) were permitted to breed in four consecutive reproductive bouts on 15-g carcasses. After 48 h, each female or pair was transferred to a new box along with its carcass. In the previous containers that contained the eggs, we placed small pieces of mouse carrion. Because larvae that hatch in the soil are attracted to carrion, this procedure enabled us to determine the number of hatched larvae and thus, the number of viable eggs laid. The boxes were checked for the presence of newly hatched larvae every 12 h. Once we observed larvae, we again transferred the beetles to a new box and placed 30 larvae on the carcass with the respective single or paired female. The old box was again provided with a small piece of carrion to collect newly hatched larvae. Once females are caring for larvae, they cease laying any additional eggs;Citation27 hence, clutch size was defined as the sum of eggs laid until the first larva hatched, specifically, the number of larvae that hatched from these eggs. To standardize the period of parental care and therefore the length of pheromone emission, pairs and singles were left to care for the larvae for a defined period of 72 h. After this time, the beetles were separated from the carcass and transferred to a new box. After being given a rest period of three days, beetles in both treatments were provided with a new carcass, and the cycle was repeated.
Twenty-four hours before each reproductive bout, single females were kept together with a male for one day to ensure their insemination. Hence, each single female was kept with a male partner for four days in their lifetime.
After the four reproductive bouts, females of both treatment groups were kept singly in small plastic containers filled with moist peat and fed mealworms twice a week until they died. In addition to the two treatment groups, we established a third group of beetles (n = 20) to investigate the effect of reproduction and parental care on longevity. In this group, females were kept singly throughout their lifetime and were never allowed to reproduce.
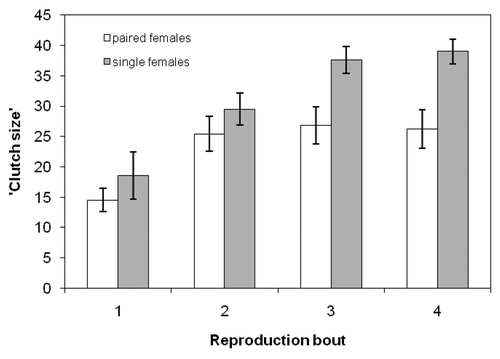
The clutch size of the beetles depended on reproductive bout (repeated measures ANOVA, F3, 75 = 20.71, p < 0.001; ). The clutch size in the first reproductive bout was smaller than those of the remaining three reproductive bouts (reproductive bout 1 vs. 2, 3, or 4, all p < 0.05). The family status of the females also had an effect on the number of eggs laid, with single females having significantly larger clutch sizes than paired females (F1, 25 = 7.91, p < 0.01; ). There was a significant interaction between reproductive bout and treatment in their effect on clutch size (F3, 75 = 5.28, p < 0.01). There was no difference in the number of eggs laid by single and paired females in the first two reproductive bouts (p > 0.05), but single females laid more eggs than females reproducing with a male partner in each of the last two reproductive bouts (each p < 0.05).
Figure 1. Clutch sizes (mean ± SE) of paired and single females in four subsequent reproductive bouts. Clutch size was determined by the number of hatched larvae.
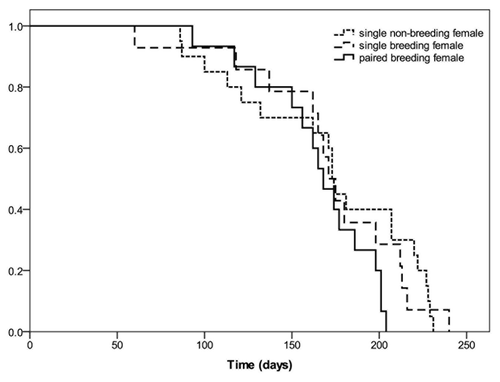
There was no difference in survival between single breeding females and paired breeding females (). Moreover, reproduction and parental care had no effect on survival, as females reproducing four times on a carcass did not die sooner than females prevented from reproducing ().
Figure 2. Cumulative survival for females that had never bred on a carcass (n = 20), bred four times without (n = 15) or with a male partner (n = 15). There was no difference in survival rates between the three treatment groups (log rank test, = 3.16, p = 0.206).
In contrast to the study by Johansson et al.,Citation10 we did not find that a higher investment in pheromone production resulted in shorter life spans. However, our results suggest a reproductive cost of chemical signaling in burying beetles: paired females produced fewer eggs in subsequent reproductive bouts than single females. A reduced fecundity as a result of increased pheromone release was also found in female moths Lobesia botrana.Citation28 In the case of N. vespilloides females, a possible explanation might be that the production of pheromones and the production of hormones regulating egg production have to compete for the same precursors. In a number of species, juvenile hormone (JH) is required for vitellogenesis and oviposition,Citation29 and burying beetles are characterized by a large and rapid increase in JH III titer shortly after the discovery of a carcass before oviposition occurs.Citation30 Although direct evidence for a gonadotropic role of JH III in burying beetles is lacking, application of JH III to food-deprived N. orbicollis results in an increased clutch size.Citation31 The pheromone methyl geranate and the hormone JH III are both terpenoids and hence, utilize the same precursors.Citation32 The higher amount of methyl geranate released by paired females might have resulted in a shortfall of precursors for JH III at the beginning of a subsequent reproductive bout, leading to a reduced clutch size in comparison to single females. However, although this explanation seems plausible, there is an alternative interpretation for our results that cannot be excluded: the difference between our two treatment groups, paired and single females, is not only the amount of methyl geranate emission, but also the presence or absence of a male partner. We know from many studies that numerous copulations can result in costs to females.Citation33-Citation36 Hence, a reduction in clutch size could be caused by a sexual conflict over mating frequency. Disentangling the costs of mating and the costs of pheromone emission on reproductive output must, however, await future study. We recommend that future investigations focus on the metabolic pathways of signal production as this would aid in identifying the exact nature of the strategic or efficacy costs of chemical signals.
Acknowledgments
We thank Scott K. Sakaluk for helpful comments on the manuscript. S.S. was supported by a Feodor Lynen Return Fellowship from the Alexander von Humboldt Foundation.
References
- Maynard-Smith J, Harper D. Animal Signals. New York: Oxford University Press 2003.
- Godfray HCJ. Signalling of need by offspring to their parents. Nature 1991; 352:328 - 30; http://dx.doi.org/10.1038/352328a0
- Zahavi A. Mate selection - Selection for a handicap. J Theor Biol 1975; 53:205 - 14; http://dx.doi.org/10.1016/0022-5193(75)90111-3; PMID: 1195756
- Zahavi A. The cost of honesty (Further remarks on the handicap principle). J Theor Biol 1977; 67:603 - 5; http://dx.doi.org/10.1016/0022-5193(77)90061-3; PMID: 904334
- Grafen A. Biological signals as handicaps. J Theor Biol 1990; 144:517 - 46; http://dx.doi.org/10.1016/S0022-5193(05)80088-8; PMID: 2402153
- Lachmann M, Szamado S, Bergstrom CT. Cost and conflict in animal signals and human language. Proc Natl Acad Sci USA 2001; 98:13189 - 94; http://dx.doi.org/10.1073/pnas.231216498; PMID: 11687618
- Számadó S. The cost of honesty and the fallacy of the handicap principle. Anim Behav 2011; 81:3 - 10; http://dx.doi.org/10.1016/j.anbehav.2010.08.022
- Ryan MJ, Cummings ME. Animals signals and the overlooked costs of efficacy. Evolution 2005; 59:1160 - 1
- Hendrichs MA, Hendrichs J. Perfumed to be killed: Interception of Mediterranean fruit fly (Diptera: Tephritidae) sexual signaling by predatory foraging wasps (Hymenoptera: Vespidae). Ann Entomol Soc Am 1998; 91:228 - 34
- Johansson BG, Jones TM, Widemo F. Cost of pheromone production in a lekking Drosophila. Anim Behav 2005; 69:851 - 8; http://dx.doi.org/10.1016/j.anbehav.2004.08.007
- Rantala MJ, Vainikka A, Kortet R. The role of juvenile hormone in immune function and pheromone production trade-offs: a test of the immunocompetence handicap principle. Proc Biol Sci 2003; 270:2257 - 61; http://dx.doi.org/10.1098/rspb.2003.2472; PMID: 14613612
- Hatano E, Kunert G, Weisser WW. Aphid wing induction and ecological costs of alarm pheromone emission under field conditions. PLoS ONE 2010; 5:e11188; PMID: 20585639
- Gosling LM, Roberts SC, Thornton EA, Andrew MJ. Life history costs of olfactory status signalling in mice. Behav Ecol Sociobiol 2000; 48:328 - 32; http://dx.doi.org/10.1007/s002650000242
- Shaver TN, Lingren PD, Marshall HF. Nighttime variation in volatile content of flowers of the night blooming plant Gaura drummondii.. J Chem Ecol 1997; 23:2673 - 82; http://dx.doi.org/10.1023/A:1022550607873
- Mazor M, Dunkelblum E. Circadian rhythms of sexual behavior and pheromone titers of two closely related moth species Autographa gamma and Cornutiplusia circumflexa.. J Chem Ecol 2005; 31:2153 - 68; http://dx.doi.org/10.1007/s10886-005-6082-7; PMID: 16132217
- Müller JK, Eggert AK. Effects of carrion-independent pheromone emission by male burying beetles (Silphidae: Necrophorus). Ethology 1987; •••:76
- Appelt CW, Sorensen PW. Female goldfish signal spawning readiness by altering when and where they release a urinary pheromone. Anim Behav 2007; 74:1329 - 38; http://dx.doi.org/10.1016/j.anbehav.2007.02.032
- Steiner S, Ruther J. Mechanism and behavioral context of male sex pheromone release in Nasonia vitripennis.. J Chem Ecol 2009; 35:416 - 21; http://dx.doi.org/10.1007/s10886-009-9624-6; PMID: 19390897
- Verheggen FJ, Haubruge E, De Moraes CM, Mescher MC. Social enviroment influences aphid production of alarm pheromone. Behav Ecol 2009; 20:283 - 8; http://dx.doi.org/10.1093/beheco/arp009
- Sakaluk SK. 1990 Sexual selection and predation: balancing reproductive and survival needs. In Insect Defenses: Adaptive Mechanisms and Strategies of Prey and Predators. (ed. D.L. Evans and J.O. Schmidt), p. 63-90. Albany, NY: SUNY Press.
- Miklas N, Lasnier T, Renou M. Male bugs modulate pheromone emission in response to vibratory signals from conspecifics. J Chem Ecol 2003; 29:561 - 74; http://dx.doi.org/10.1023/A:1022898620429; PMID: 12757319
- Steiger S, Haberer W, Müller JK. Social environment determines degree of chemical signalling. Biol Lett 2011; •••; http://dx.doi.org/10.1098/rsbl.2011.0457; PMID: 21653566
- Pukowski E. Ökologische Untersuchungen an Necrophorus F. Z Morphol Oekol Tiere 1933; 27:518 - 86; http://dx.doi.org/10.1007/BF00403155
- Haberer W, Steiger S, Müller JK. (E)-Methylgeranate, a chemical signal of juvenile hormone titre and its role in the partner recognition system of burying beetles. Anim Behav 2010; 79:17 - 24; http://dx.doi.org/10.1016/j.anbehav.2009.09.019
- Müller JK, Eggert AK, Elsner T. Nestmate recognition in burying beetles: The “breeder's badge” as a cue used by females to distinguish their mates from male intruders. Behav Ecol 2003; 14:212 - 20; http://dx.doi.org/10.1093/beheco/14.2.212
- Trumbo ST. Reproductive benefits of infanticide in a biparental burying beetle Nicrophorus orbicollis.. Behav Ecol Sociobiol 1990; 27:269 - 74; http://dx.doi.org/10.1007/BF00164899
- Müller JK, Eggert AK, Furlkroeger E. Clutch size regulation in the burying beetle Necrophorus vespilloides Herbst Coleoptera Silphidae. J Insect Behav 1990; 3:265 - 70; http://dx.doi.org/10.1007/BF01417917
- Harari AR, Zahavi T, Thiery D. Fitness cost of pheromone production in signaling female moths. Evolution 2011; 65:1572 - 82; http://dx.doi.org/10.1111/j.1558-5646.2011.01252.x; PMID: 21644949
- Koeppe J, Fuchs M, Chen T, Hunt L, Kovalick G, Briers T. 1985 The role of juvenile hormone in reproduction. In Comprehensive Insect Physiology, Biochemistry and Pharmacology (ed. G. Kerkut and L. Gilbert), p. 165-204. Oxford: Pergamon.
- Scott MP, Trumbo ST, Neese PA, Bailey WD, Roe RM. Changes in biosynthesis and degradation of juvenile hormone during breeding by burying beetles: A reproductive or social role?. J Insect Physiol 2001; 47:295 - 302; http://dx.doi.org/10.1016/S0022-1910(00)00116-5; PMID: 11119775
- Trumbo ST, Robinson GE. Nutrition, hormones and life history in burying beetles. J Insect Physiol 2004; 50:383 - 91; http://dx.doi.org/10.1016/j.jinsphys.2004.01.008; PMID: 15121451
- Bellés X, Martin D, Piulachs MD. The mevalonate pathway and the synthesis of juvenile hormone in insects. Annu Rev Entomol 2005; 50:181 - 99; http://dx.doi.org/10.1146/annurev.ento.50.071803.130356; PMID: 15355237
- Chapman T, Liddle LF, Kalb JM, Wolfner MF, Partridge L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 1995; 373:241 - 4; http://dx.doi.org/10.1038/373241a0; PMID: 7816137
- Chapman T, Arnqvist G, Bangham J, Rowe L. Sexual conflict. Trends Ecol Evol 2003; 18:41 - 7; http://dx.doi.org/10.1016/S0169-5347(02)00004-6
- Stutt AD, Siva-Jothy MT. Traumatic insemination and sexual conflict in the bed bug Cimex lectularius. Proc Natl Acad Sci USA 2001; 98:5683 - 7; http://dx.doi.org/10.1073/pnas.101440698; PMID: 11331783
- Sakaluk SK, Avery RL, Weddle CB. Cryptic sexual conflict in gift-giving insects: Chasing the chase-away. Am Nat 2006; 167:94 - 104; http://dx.doi.org/10.1086/498279; PMID: 16475102