Abstract
Meiosis is a specialized cell division process by which diploid germ line cells generate haploid gametes, which are required for sexual reproduction. During this process, several micronutrients are required, including copper ions. Despite important roles for copper-dependent proteins during meiosis, their mechanisms of action remain poorly understood. In a recently publication, we reported the discovery of Mfc1, the first example ever reported of a meiosis-specific copper transporter. Although Mfc1 did not exhibit any significant amino acid sequence similarities with members of the Ctr family of copper transporters, it harbored putative copper coordination motifs. Microarray data showed that mfc1+ was the most highly induced of all meiotic genes detected under copper-limiting conditions. Analysis of Mfc1 localization during meiosis revealed that it localized at the forespore membrane during middle and late phases of the meiotic program. Interestingly, live-cell copper imaging using a copper-binding tracker revealed accumulation of copper ions into the forespore in wild-type cells. In contrast, mutant cells lacking Mfc1 displayed an intracellular distribution of copper ions that was dispersed throughout the ascospores without any marked preference for the forespore. We propose that Mfc1 is required to mobilize copper into the forespore, thereby providing copper to copper-requiring enzymes of the developing spores.
Redox active transition metals such as copper present a dilemma to cells. Although they are essential cofactors, they can also be cytotoxic.Citation1 On the one hand, copper serves as a catalytic and a structural cofactor of many enzymes but, on the other hand, due to its proclivity to change redox state, it can react with hydrogen peroxide to generate the highly toxic hydroxyl radical.Citation2 Consequently, it is critical that cells use homeostatic mechanisms to acquire and maintain sufficient amounts of copper while at the same time preventing its accumulation to protect the cells from its unwanted toxic effects.
The copper homeostatic machinery has traditionally been investigated in dividing cells that grow mitotically (vegetative growth). Although deficiencies in copper-dependent proteins culminate in meiotic cell developmental defects and subfertility,Citation3,Citation4 copper homeostasis has been poorly studied during meiosis. Contributing reasons for that situation include: (1) paucity of mammalian germ cells, (2) unavailability of germ cell lines grown under copper depleted or copper sufficient conditions, (3) heterogeneity of mammalian germ cell populations and, (4) lack of information on the number and nature of molecules that serve as potential players, especially in response to changes in copper levels during meiosis.Citation5 To gain insight into the molecular basis of copper homeostasis during meiosis, we have combined the use of DNA microarrays and the fission yeast Schizosaccharomyces pombe. The fission yeast is one of the best-understood model systems to investigate the eukaryotic cell cycle, either the conventional mode of division (mitosis) or the meiotic cell division program (meiosis).Citation6 S. pombe is also of special interest because fission yeast cells possess similarities to mammalian cells in several respects. These include the mode of cell division (septation/medial cleavage) and the regulation of the cell-cycle (Cdc-like proteins). This latter property is especially relevant to meiosis, since it can be followed from its initiation through to generation of mature haploid cells via highly conserved meiotic proteins (Spo11, Sgo1 and Rec8).Citation7 In addition, S. pombe has become particularly attractive for the study of key molecular aspects of meiosis because conditional-growth and temperature-sensitive mutants have been developed that allow synchronization of the cells prior to entry into the meiotic program. This latter point is of paramount importance since animal models (vitamin A-deficient mice) and tissue co-cultured cells (Sertoli cells with germ cells) are not easy to synchronize prior to their entry into meiosis.Citation8,Citation9
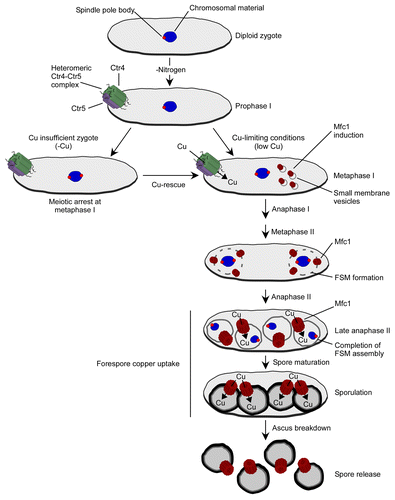
In our recent publication, we have used the S. pombe model to study meiosis. We have discovered that copper was absolutely required for progression of meiosis because copper insufficient zygotes exhibited a meiotic arrest at metaphase I ().Citation10 During early meiosis, copper uptake is most likely ensured by the heteromeric Ctr4-Ctr5 complex as the Ctr4 protein localizes at the cell surface of developing asci and remains at the plasma membrane until the 3 h meiotic time point.Citation10 This preferred location of the Ctr4-Ctr5 copper-transport system coincides primarily with the start of meiosis and the premeiotic S-phase and recombination. However, when middle meiosis is initiated, Ctr4 expression is abolished. To follow up on this observation, microarrays were hybridized with probes derived from RNA isolated from copper-starved vs. copper-replete meiotic cells. At middle meiosis, analysis of gene expression profiling data identified several uncharacterized genes, including the SPAPB1A11.01 gene, which was highly transcriptionally induced in copper-starved cells. We named this novel SPAPB1A11.01 gene mfc1+. Further investigations revealed that mfc1+ exhibited a distinct temporal expression profile when compared with that of ctr4+, since it was expressed after 3 h of meiotic induction. The levels of mfc1+ mRNA reached a maximum within 5 h, coinciding with meiotic divisions. Thereafter, the expression profile of mfc1+ was relatively sustained with only a slight decrease over time being observed.Citation10
Figure 1. A model for copper transport during meiosis in S. pombe. A zygote under low nitrogen (N) and copper (Cu) conditions proceeds through meiosis. In contrast, a copper insufficient zygote displays a meiotic arrest at metaphase I. At early stages of meiosis (prophase and metaphase I), copper uptake is mediated by the heteromeric Ctr4-Ctr5 complex (green and violet cylinders). During metaphase I, while the Ctr4-Ctr5 heterocomplex disappears from the cell surface, Mfc1 (brown cylinders) is first detected in small membranous vesicle structures within the cytoplasm of zygotic cells. These membranous vesicles appear to fuse to subsequently form the forespore membrane (FSM). After FSM closure, Mfc1 resides at the FSM where it persists until matured spores are released from the ascus. Blue, chromosomal material; red, spindle pole body; gray, FSM.
Under conditions of copper starvation, the meiotic transcriptional program revealed that ctr4+ and mfc1+ transcript levels were induced at distinct times during meiosis. Whereas the deletion of the cuf1+-encoded copper-dependent transcription factor (cuf1Δ mutant) impaired the induction of ctr4+, transcriptional activation of mfc1+ was unaffected. This observation suggested the existence of a distinct transcriptional regulator of induction of mfc1+ in response to copper starvation.Citation10 This result was also consistent with the fact that only a single, inverted weak putative copper-signaling element (CuSE) was identified in the promoter region of mfc1+.Citation10,Citation11 Based on these observations, it can be suggested that the DNA sequence requirement for copper starvation-dependent induction of mfc1+ is different than that of the ctr+ genes, including ctr4+, ctr5+ and ctr6+. Global transcriptome and deletome profiles of seven meiosis-specific transcription factors that are known to be essential for successful meiotic differentiation and sporulation have consistently shown that the mfc1+ gene was not regulated by Rep1, Mei4, Cuf2, Atf21, Atf31, Rsv1 and Rsv2 (unpublished data).Citation12 Again, these results represent compelling arguments in favor of the interpretation that an uncharacterized meiotic regulator is responsible for copper-dependent regulation of the mfc1+ gene.
During middle meiosis, a Cherry epitope-tagged Mfc1 protein was first detected on membranes of small intracellular vesicles that are thought to originate from the endoplasmic reticulum and Golgi apparatus network.Citation10,Citation13 These intracellular vesicles that are known to fuse for the assembly of the forespore membrane (FSM) carry integral transmembrane proteins needed for the FSM maturation and function. One of the vesicle-trafficking pathways involves many proteins, including Spo14 and Spo20. Based on sequence homology data, spo14+ encodes an ortholog of S. cerevisiae Sec12, which is a GDP/GTP exchange factor for the GTP-binding protein Sar1 that is required for vesicle trafficking from the ER to the Golgi.Citation14 The Spo20 protein is highly similar to S. cerevisiae Sec14, which is a phosphatidyl choline/phosphatidyl inositol-transfer protein.Citation15 In budding yeast, Sec14 is essential for vesicle budding from the Golgi apparatus.Citation16 According to a proposed model, after fusion of membrane vesicles to form FSM, the Mfc1-Cherry fluorescent protein was observed in the surroundings of FSM. Indeed, Mfc1-Cherry-associated fluorescence produced four fluorescent circle-like structures that corresponded to precursor spores.Citation10 Mfc1 is expressed exclusively during meiosis, whereas other FSM resident proteins such as Psy1 are expressed during the two types of cell cycle programs.Citation13 When Psy1 is expressed in mitotically growing cells, it localizes to the plasma membrane.Citation13 However, when cells undergo meiosis, Psy1 disappears from the plasma membrane after the first meiotic division and relocalizes to the nascent FSM as the second meiotic division starts. The relocation of Psy1 from the plasma membrane to FSM may use a distinct intracellular route as opposed to Mfc1. Further analysis of genes (eg. spo14+, spo20+) that encode putative components of vesicle-trafficking pathways will be required to decipher the relocation mechanism of Psy1 and Mfc1 during the “switching-on” of de novo membrane formation at the FSM.
Over the past years, studies have suggested the presence of independent activities for copper transport within cells.Citation17 Frequently, cells possess both primary and secondary active transporters for the same substrate. Whereas primary transporters bind ATP and require ATP hydrolysis for transport activity, secondary transporters are driven by proton-motive or gradient forces.Citation18 Thus far, copper is known to be transported across membranes by at least two types of transmembrane proteins. These are the P-type ATPases and the Ctr (channel-like) proteins.Citation1 Based on computer analysis, Mfc1 is predicted to be related to the major facilitator superfamily (MFS) of transporters, which is the largest group of secondary membrane transporters.Citation19 Mfc1 contains 19 Met residues, four pairs of which are present in the potential copper coordination arrangements Met-X-Met or Met-X2-Met.Citation20 Furthermore, Mfc1 has 7 Cys residues scattered throughout the protein that are potential copper-binding ligands. These observations suggest that Mfc1 may bind copper through these amino acid residues, and this may also be an important feature related to its activity. In the context of a global analysis of protein localization in S. pombe, a GFP epitope-tagged mfc1+ allele has been artificially expressed in cells proliferating in mitosis.Citation21 In these cells, the mfc1+-gfp gene product localized at the plasma membrane. To evaluate the potential contribution of Mfc1 in copper acquisition, ctr4Δ ctr5Δ cells expressing the mfc1+-gfp gene under control of a heterologous mitotic promoter were assayed for respiratory competence and results compared with a wild-type strain harboring the ctr4+ and ctr5+ genes. ctr4Δ ctr5Δ cells failed to grow on a nonfermentable carbon source due to their inability to provide copper to the copper-requiring cytochrome c oxidase enzyme. When Mfc1-GFP was expressed at the plasma membrane in mitotic cells grown in a medium containing 2 μM CuSO4, copper was delivered to cytochrome c oxidase, allowing cells to grow on respiratory carbon sources.Citation10 Furthermore, 64Cu uptake assays in ctr4Δ ctr5Δ cells expressing the mfc1+-gfp allele revealed that these cells had the property to transport 64Cu with high affinity in a manner that was consistent with their capacity to utilize nonfermentable carbon sources for growth.Citation10
In meiosis, the subcellular localization of Mfc1 at FSM suggested that Mfc1 was an intracellular transporter that mediated copper uptake into the forespore. This finding suggested the presence of a pathway by which copper is transported into this compartment where copper is required for copper-dependent enzyme activities. This proposed function of Mfc1 is supported by the fact that deletion of the mfc1+ gene (mfc1Δ/Δ) resulted in a strong reduction in copper amine oxidase 1 activity, a cuproenzyme found in the forespore.Citation10 Furthermore, as shown by experiments using Coppersensor-1 (CS1),Citation22 analysis of live-cell labile copper in a mfc1Δ/mfc1Δ mutant revealed that labile copper pools were mainly detected outside the forespores.Citation10 In contrast, wild-type cells (mfc1+/mfc1+) exhibited an intense punctuate fluorescence corresponding to the presence of labile copper pools within the forespores. The fluorescent detection of labile copper pools in forespores in mfc1+/mfc1+ cells adds further weight to the argument favoring a role for Mfc1 in mediating copper uptake into forespores. As all of the proteins identified in copper transport in yeast have higher eukaryote homologs, it is not surprising that we have recently found a putative mouse ortholog of Mfc1 (denoted as mMfc1). Using typical copper acquisition assays, our preliminary results have revealed that the mMfc1 protein functionally complements yeast cells defective in copper transport (unpublished data). Consequently, our discovery of the involvement of Mfc1 in copper transport in S. pombe opens the way for further investigations of the role of Mfc1 and its putative homologs in physiology and copper homeostasis in gamete biology.
| Abbreviations: | ||
| CAO | = | copper amine oxydase |
| Cherry | = | red fluorescent protein |
| Coppersensor-1 | = | CS1 |
| Ctr | = | copper transporter |
| Cu | = | copper |
| Cuf1 | = | copper factor 1 |
| Cuf2 | = | copper factor 2 |
| CuSE | = | copper-signaling element |
| FSM | = | forespore membrane |
| GFP | = | green fluorescent protein |
| Mfc1 | = | major facilitator copper transporter 1 |
| MFS | = | major facilitator superfamily |
| N | = | nitrogen |
| WT | = | wild-type |
Acknowledgment
We are grateful to Dr. Gilles Dupuis for critically reading the manuscript and for his valuable comments. R.I. is recipient of a studentship from the Natural Sciences and Engineering Research Council of Canada (NSERC). This work was supported in part by grants MOP3645 and MOP114986 from the Canadian Institutes of Health Research (CIHR) and by a grant from the Foundation of Stars for Children’s Health Research awarded to S.L.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol 2008; 4:176 - 85; http://dx.doi.org/10.1038/nchembio.72; PMID: 18277979
- Boal AK, Rosenzweig AC. Structural biology of copper trafficking. Chem Rev 2009; 109:4760 - 79; http://dx.doi.org/10.1021/cr900104z; PMID: 19824702
- Narisawa S, Hecht NB, Goldberg E, Boatright KM, Reed JC, Millan JL. Testis-specific cytochrome c-null mice produce functional sperm but undergo early testicular atrophy. Mol Cell Biol 2002; 22:5554 - 62; http://dx.doi.org/10.1128/MCB.22.15.5554-5562.2002; PMID: 12101247
- Kowal M, Lenartowicz M, Pecio A, Golas A, Blaszkiewicz T, Styrna J. Copper metabolism disorders affect testes structure and gamete quality in male mice. Syst Biol Reprod Med 2010; 56:431 - 44; http://dx.doi.org/10.3109/19396361003734624; PMID: 20849226
- Kim AM, Vogt S, O'Halloran TV, Woodruff TK. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat Chem Biol 2010; 6:674 - 81; http://dx.doi.org/10.1038/nchembio.419; PMID: 20693991
- Harigaya Y, Yamamoto M. Molecular mechanisms underlying the mitosis-meiosis decision. Chromosome Res 2007; 15:523 - 37; http://dx.doi.org/10.1007/s10577-007-1151-0; PMID: 17674143
- Marston AL, Amon A. Meiosis: Cell-cycle controls shuffle and deal. Nat Rev Mol Cell Biol 2004; 5:983 - 97; http://dx.doi.org/10.1038/nrm1526; PMID: 15573136
- Nagy A, Gertsenstein M, Vintersten K, Behringer R, eds. Manipulating the mouse embryo: A laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, USA, 2003.
- Staub C. A century of research on mammalian male germ cell meiotic differentiation in vitro. J Androl 2001; 22:911 - 26; PMID: 11700854
- Beaudoin J, Ioannoni R, López-Maury L, Bähler J, Ait-Mohand S, Guérin B, et al. Mfc1 is a novel forespore membrane copper transporter in meiotic and sporulating cells. J Biol Chem 2011; 286:34356 - 72; http://dx.doi.org/10.1074/jbc.M111.280396; PMID: 21828039
- Beaudoin J, Labbé S. The fission yeast copper-sensing transcription factor Cuf1 regulates the copper transporter gene expression through an Ace1/Amt1-like recognition sequence. J Biol Chem 2001; 276:15472 - 80; http://dx.doi.org/10.1074/jbc.M011256200; PMID: 11278870
- Mata J, Wilbrey A, Bähler J. Transcriptional regulatory network for sexual differentiation in fission yeast. Genome Biol 2007; 8:R217; http://dx.doi.org/10.1186/gb-2007-8-10-r217; PMID: 17927811
- Shimoda C, Nakamura T. Control of late meiosis and ascopore formation. pp. 311-27. In The Molecular Biology of Schizosaccharomyces pombe, (Egel, R., ed), Springer-Verlag, Berlin, Germany, 2004.
- Nakamura-Kubo M, Nakamura T, Hirata A, Shimoda C. The fission yeast spo14+ gene encoding a functional homologue of budding yeast Sec12 is required for the development of forespore membranes. Mol Biol Cell 2003; 14:1109 - 24; http://dx.doi.org/10.1091/mbc.E02-08-0504; PMID: 12631727
- Nakase Y, Nakamura T, Hirata A, Routt SM, Skinner HB, Bankaitis VA, et al. The Schizosaccharomyces pombe spo20+ gene encoding a homologue of Saccharomyces cerevisiae Sec14 plays an important role in forespore membrane formation. Mol Biol Cell 2001; 12:901 - 17; PMID: 11294895
- Bankaitis VA, Malehorn DE, Emr SD, Greene R. The Saccharomyces cerevisiae SEC14 gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast golgi complex. J Cell Biol 1989; 108:1271 - 81; http://dx.doi.org/10.1083/jcb.108.4.1271; PMID: 2466847
- Lee J, Petris MJ, Thiele DJ. Characterization of mouse embryonic cells deficient in the ctr1 high affinity copper transporter. Identification of a Ctr1-independent copper transport system. J Biol Chem 2002; 277:40253 - 9; http://dx.doi.org/10.1074/jbc.M208002200; PMID: 12177073
- Pao SS, Paulsen IT, Saier MH Jr.. Major facilitator superfamily. Microbiol Mol Biol Rev 1998; 62:1 - 34; PMID: 9529885
- Law CJ, Maloney PC, Wang DN. Ins and outs of major facilitator superfamily antiporters. Annu Rev Microbiol 2008; 62:289 - 305; http://dx.doi.org/10.1146/annurev.micro.61.080706.093329; PMID: 18537473
- Puig S, Lee J, Lau M, Thiele DJ. Biochemical and genetic analyses of yeast and human high affinity copper transporters suggest a conserved mechanism for copper uptake. J Biol Chem 2002; 277:26021 - 30; http://dx.doi.org/10.1074/jbc.M202547200; PMID: 11983704
- Matsuyama A, Arai R, Yashiroda Y, Shirai A, Kamata A, Sekido S, et al. ORFeome cloning and global analysis of protein localization in the fission yeast Schizosaccharomyces pombe. Nat Biotechnol 2006; 24:841 - 7; http://dx.doi.org/10.1038/nbt1222; PMID: 16823372
- Zeng L, Miller EW, Pralle A, Isacoff EY, Chang CJ. A selective turn-on fluorescent sensor for imaging copper in living cells. J Am Chem Soc 2006; 128:10 - 1; http://dx.doi.org/10.1021/ja055064u; PMID: 16390096