Abstract
Henningsmoenicaris scutula (Walossek and Müller, 1990) () is a tiny representative of Crustacea, systematically standing close to the stemline. It is found in stinkstone (‘Orsten’) nodules from the Alum Shale, where a rich fauna of small organisms is excellently preserved. Three dimensional morphology is retained by phosphatisation, which exhibits the finest details, such as cuticular structures, fine appendages and especially the morphology of the compound eyes. The stalked eyes of H. scutula investigated here were equipped with a differentiated visual surface with four different areas of vision. The most intriguing is a field of view oriented laterally to the contralateral side of each eye, so that the fields of view of both compound eyes intersect, and give information about any object moving within the vicinity. Due to this, although, for various reasons this compound eye probably was not able to form a proper image, it was able to perceive tiny prey within a wide visual field, in the same way that the movement of figures can be traced in a chess game. This can be considered as a highly sophisticated visual system that developed early in the history of reported eye evolution, as this compound eye is almost exactly half a billion years old.
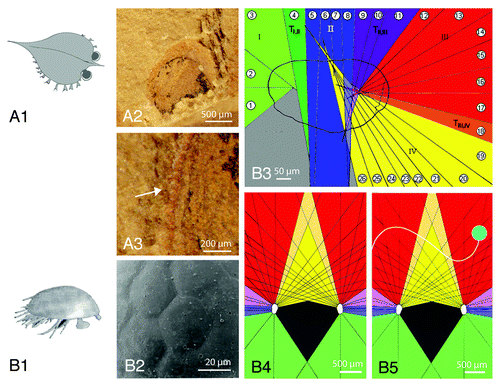
Figure 1. Types of Cambrian Eyes and the functioning of the eye of Henningsmoenicaris scutula (Waklossek and Müller, 1990).Citation6 (A1) Schematic drawing of Isoxys auritus Jiang, 1982, a pelagic arthropod from the oldest fossil record of arthropods (Chengjiang Lagerstätte, Lower Cambrian, Yunnan, China). Note the stalked eyes protruding from the carapace, and covered by a cuticularian top, the palpebral lobe. (A2) Indvidual eye of I. auritus, seen from above. Note the palpebral lobe and the visual surface pressed out sidewards when being embedded. (A3) Spherical visual units visible at the margin of the palpebral lobe, seen from above, indicated by the arrow. (B1) Schematic drawing of H. scutula (Waklossek and Müller, 1990),Citation6 reconstruction after Haug (Haug et al. 2009).Citation8 (B2) The stalked eyes of H. scutula, like many ‘Orsten’ fossil eyes show well preserved facets. (B3) Distinct visual areas of H. scutula within one eye, defined by intersections (‘focal points’) of the optical axes of the ommatidia. The numbers indicate the opening angles of the ommatidia in longitudinal projection. (B3) visual field of H. scutula. Note the intersections of the optical axes of the ommatidia (black lines) which intersect in the front of the organism and far to the sides. The dark gray area indicates the ‘blind’, which subtends the shape of the body of the organism. (B5) Any moving particle can be traced in the coordinates by facets of both eyes like a figure in a chess game.
‘Orsten’ - fossils are tiny, three-dimensionally preserved organisms belonging to numerous systematic groups, known from the Lower Cambrian to Lower Ordovician from various continents (for more details see Maas et al.Citation1). Henningsmoenicaris scutula (Walossek and Müller, 1990), investigated here, was found in nodules in the quarry of Gum, at Mount Kinnekülle, Västergötland, Sweden. These nodules belong to the ‘stinkstone’ -limestone (‘Orsten’) of the still unnamed Series 3, which underlies the Furongian (499-488,3 Ma), and thus is almost exactly half a billion years old. The Furongian is the upper stratigraphic part (series) of the Cambrian, and is followed by the Ordovician, a period which brought a wide diversification of species after the vigorous (reported) diversification during the early beginnings of the Cambrian, known as the ‘Cambrian Explosion’.
The ‘Orsten’-fossils can be dissolved from their matrix by 10% acetic acid and have been investigated using the SEM. The arthropods, most of which are not larger than 2 mm, reveal finest cuticularian structures like hairs, bristles and sensilla, pores or gland openings, details which may be thinner than 0.3 µm.Citation2 This characteristic type of preservation also allows the analysis of compound eyes in great detail, and the three-dimensional preservation opens up far wider perspectives for their study than those of ‘orthodox’ modes of preservation.
Among the ‘Orsten’- fossils a wide range of arthropods is represented, such as lobopodiansCitation1 (Maas et al. 2006), tardigrades,Citation3 cheliceratesCitation4 and crustaceans.Citation2,Citation5 Some are derivatives of the stem lineage toward the Eucrustaceans (crown group)Citation6-Citation8 including H. scutula. This was interpreted as a tiny predator because the specialized appendages indicating such a life style.Citation8 An autapomorphy of Euarthropoda is the possession of compound eyes.
Compound eyes consist of identical repetitive visual units, the so-called ommatidia, each of which is orientated to a slightly different part of the environment. There exist several functional types of ommatidia, the most basal are those of the apposition eye; more derived kinds are not be found before Jurassic times.Citation9 All ommatidia of an apposition eye consist of a dioptric apparatus, which focuses the light onto a central light guiding structure, the rhabdom. This rhadom is part of the sensory cells and contains the visual pigments, which, as the light comes in, changes the chemical structure and thus evokes a slight electrical signal which is then processed by the central nervous system of the organism. Because all light which enters the ommatidium is combined in the rhabdom, no details can be distinguished by the ommatidium itself, just an average of contrasts. Thus results from the entire compound eye an overall mosaic-like image.Citation10 The acuity of this image depends on the opening angle of the ommatidia, to be more precise of the rhabdom, and the number of facets, as with pixels of a computergraphic define the fineness of vision.
The most representative owners of compound eyes in the Palaeozoic, undoubtedly are trilobites, extinct arthropods which lived from ~522 Ma to ~250 Ma. They have existed since the early Cambrian with highly developed compound eyes, often reminiscent modern facetted eyes. The earliest trilobite compound eyes date to ~522 Ma in small eodiscid trilobites from Australia and China,Citation11,Citation12 and even stalked eyes have been reported in trilobites,Citation13 which could not be moved, unlike many stalked eyes today. An excellent overview of trilobite vision is given in Clarkson et al.Citation14
The oldest non-trilobite compound eyes documented and analyzed come from the lower Cambrian Chengjiang Lagerstätte (~520 to ~525 Ma) with its sometimes excellent preservation,Citation15-Citation19 the above-mentioned eodiscid trilobite eyes are of similar age. In typical Chengjiang representatives such as Isoxys auritus Jiang, 1982 () or Fuxianhuia protensa Hou, 1987 the eyes are more or less spherical and the spherical visual units () are arranged less densely than in advanced modern arthropods, where the facets are (normally) packed hexagonally, as we know them from bees or dragonflies living today. Possibly these basal visual units are not proper ommatidia as explained before, but ocelli, which are small cups floored by sensory cells like a tiny retina. But among the Chengjiang fauna there already exist compound eyes with hexagonal densely packed arrangements of ommatidia also, as for example in Cindarella eucalla.Citation18 Recently published was a description of a slightly younger moult of a compound eye with a high number of facets from Emu Bay (Australia),Citation20 reminiscent of those of modern dragonflies, and so it was suggested to have belonged to an unknown predator. Finally, there are ‘Orsten’-fossils, from the late Cambrian, which show the finest details of all, including even three dimensional structures such as facets () (compare ref. Citation21, Fig 1C). In the ‘cyclopic’ forms such as Cambropachycope clarksoni Walossek and Müller 1990 it is even probable for example to show, that probably polarized light was used for predation, an astonishing convergence with the tactics used by living predatory waterfleas.Citation22 Even light filtering mechanisms with the cornea have been established.Citation23
Undoubtedly the development of eyes has been an effective trigger of evolution, the race between prey and predator, the struggle to survive, has forced the dynamics of evolution.Citation24,Citation25
In this context it appears remarkable that we found among the ‘Orsten’-fossils the tiny stalked eyes of H. scutula,Citation26 which exhibit joint structures at their basal ends and thus were very probably movable. The eye itself is so small, less than 400 µm, that a high-resolution visual surface was unlikely to be established, which would be crucial for a visually orientated predator. Applying theoretical tools of modern biology,Citation27-Citation29 it was possible to show that these eyes were adapted to bright light conditions. Thus the organism probably lived close to the water surface, which would be of great advantage for these very small eyes to become able to capture enough light to function. The most intriguing part of the story, however, is that the fossilised eye reveals the traces of the rhabdoms as small, deep cavities in the fossil, indicating the precise direction of the optical axis of each ommatidium. The reconstructions show that there exist several distinct functional groups of ommatidia in the visual surface (), orientated downwards and backward, laterally, and to the front, but the most conspicious is an inwardly orientated one with a high acuity. This visual system only makes functional sense if the second eye is taken into consideration (). The intersection of the visual fields of the ommatidia of both eyes can be interpreted as a kind of stereoscopic vision, which in H scutula is not just orientated to the front as in many recent arthropods, but also to the sides. These intersections produce a three-dimensional lattice, giving coordinates by the facets of each eye. Any moving particle within the visual field of both compound eyes can be traced as in the figures in a chess games (). Velocity, distances and size of (moving) particles can be made out, and even without having a detailed image, H. scutula will be informed about “what’s going on” in its visual field. A ‘blind area’, covered by no facets, indicates in recent animals the body size,Citation30 thus the size of H. scutula could be estimated to just ~1.5mm.
If these stalks are moved, so the fineness of visual perception changes, because the units of intersection change along with expansion of the field of view, and can be raised enormously.Citation21
Thus, this sophisticated coordinate system certainly supports the concept of a predatory life style, a clever and specialized visual system of a tiny eye very early in the history of the evolution of vision.
Acknowledgments
My sincere thanks to Prof. E.N.K. Clarkson for his advice and constructive discussion, and I am greatly indebted to D. Waloszek, J.T. Haug, A., Maas, C. Castellani and C. Haug for their generous support.
References
- Maas A, Braun A, Dong X-P, Donoghue P, Müller KJ, Olempska E, et al. The ‘Orsten’ – more than a Cambrian Konservat-Lagerstätte yielding exceptional preservation. Palaeoworld 2006; 15:266 - 82; http://dx.doi.org/10.1016/j.palwor.2006.10.005
- Waloszek D. The ‘Orsten’ Window – A three-dimensionally preserved upper Cambrian meiofauna and its contribution to our understanding of the evolution of arthropoda. Paleont Resh 2003; 7:71 - 88; http://dx.doi.org/10.2517/prpsj.7.71
- Müller KJ, Walossek D, Zakharov A. ‘Orsten’ type phosphatized soft-integument preservation and a new record from the middle Cambrian Kuonamka formation in Siberia. N Jb Geol Palaeont Abhandl 1995; 191:101 - 18
- Waloszek D, Dunlop J. A larval sea spider (Arthropoda: Pycnogonida) from the Upper Cambrian ‘Orsten’ of Sweden, and the phylogenetic position of pycnogonids. –. Palaeontology 2002; 45:421 - 46; http://dx.doi.org/10.1111/1475-4983.00244
- Budd GE. The earliest fossil record of the animals and its significance. Philos Trans R Soc Lond B Biol Sci 2008; 363:1425 - 34; http://dx.doi.org/10.1098/rstb.2007.2232; PMID: 18192192
- Walossek D, Müller KJ. Stem-lineage crustaceans from the Upper Cambrian of Sweden and their bearing upon the position of Agnostus. Lethaia 1990; 23:409 - 27; http://dx.doi.org/10.1111/j.1502-3931.1990.tb01373.x
- Stein M, Waloszek D, Maas A, Haug JT, Müller KJ. The stem crustacean Oelandocaris oelandica revisited. Acta Palaeontol Pol 2008; 53:461 - 84; http://dx.doi.org/10.4202/app.2008.0308
- Haug JT, Maas A, Waloszek D. Ontogeny of the stem-crustaceans Goticaris longispinosa and Cambropachycope clarksoni from the Upper Middle Cambrian ‘Orsten’ of Sweden. Palaeontographica 2009; 289:1 - 43
- Gaten E. Eye structure and phylogeny: is there an insight? The evolution of superposition eyes in the Decapoda (Crustacea). Contrib Zool 1998; 67:223 - 35
- Müller J. Zur vergleichenden Physiologie des Gesichtssinnes des Menschen und der Thiere. Cnobloch, Leipzig, 1826.
- Jell PA. The abathochroal eye of Pagetia, a new type of trilobite eye. Fossils and Strata 1975; 4:33 - 43
- Zhang X-G, Clarkson ENK. The eyes of Lower Cambrian eodiscid trilobites. Palaeontology 1990; 33:911 - 32
- Peng S-c, Yang X, Hughes NC. The oldest stalk-eyed trilobite, Parablackwelderia Kobayashi, 1942 (Damesellinae, Cambrian), and its occurrence in Shandong, China. J Paleontol 2008; 82:842 - 50; http://dx.doi.org/10.1666/07-123.1
- Clarkson ENK, Levi-Setti R, Horváth G. The eyes of trilobites: The oldest preserved visual system. Arthropod Struct Dev 2006; 35:247 - 59; http://dx.doi.org/10.1016/j.asd.2006.08.002; PMID: 18089074
- Schoenemann B. Cambrian View. Palaeoworld 2006; 15:307 - 14; http://dx.doi.org/10.1016/j.palwor.2006.10.012
- Schoenemann B. Analyse früher Augensysteme. Freiberger Forschungsberichte 2008; 15:85 - 96
- Schoenemann B, Clarkson ENK. The eyes of Isoxys – Eye morphology indicates the ecological habitat of an a half million year old animal. Lethaia 2010; 44:223 - 30; http://dx.doi.org/10.1111/j.1502-3931.2010.00239.x
- Schoenemann B, Clarkson ENK. At First Sight – Functional Analysis of Lower Cambrian Eye Systems. Palaeontographica A 2011; 4: in review; In review
- Schoenemann B, Clarkson ENK. The eyes of Leanchoilia. Lethaia 2011; In press
- Lee MSY, Jago JB, García-Bellido DC, Edgecombe GD, Gehling JG, Paterson JR. Modern optics in exceptionally preserved eyes of Early Cambrian arthropods from Australia. Nature 2011; 474:631 - 4; http://dx.doi.org/10.1038/nature10097; PMID: 21720369
- Schoenemann B, Castellani C, Clarkson ENK, Haug JT, Maas A, Haug C, et al. The sophisticated visual system of a tiny Cambrian crustacean: analysis of a stalked fossil compound eye. Proc Biol Sci 2011; 279:1335 - 40; http://dx.doi.org/10.1098/rspb.2011.1888; PMID: 22048954
- Schoenemann B, Haug JT, Parker AR, Waloszek D, Maas A, Castellani C, Haug C. Was polarised light used by Cambrian micro-predators? Astonishing convergences to modern raptorial waterfleas. 2012; in preparation.
- Parker AR, Schoenemann B, Haug JT, Waloszek D. An unusual cornea from a well- preserved (‘Orsten’) Cambrian compound eye; In preparation.
- Parker AR. Colour in Burgess Shale animals and the effect of light on evolution in the Cambrian. Proc Biol Sci 1998; 265:967 - 72; http://dx.doi.org/10.1098/rspb.1998.0385
- Parker AR. In the Blink of an Eye. London: Simon and Schuster, 2003.
- Castellani C, Haug TH, Haug C, Maas A, Schoenemann B, Waloszek D. Exceptionally well- preserved isolated eyes from Cambrian, ‘Orsten’ fossil assemblages of Sweden. Palaeontology 2011; In press
- Horridge GA. Insects which turn and look. Endeavour 1977; 1:7 - 17; http://dx.doi.org/10.1016/0160-9327(77)90004-7
- Snyder AW. The acuity of compound eyes: physical limitations and design. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 1977; 116:161 - 82; http://dx.doi.org/10.1007/BF00605401
- Land MF. Optics and vision in invertebrates. In: Autrum H, ed. Handbook of Sensory Physiology, Vol. VII/6B. Berlin: Springer-Verlag, 1981; 471-92
- Burkhardt D. 1973 Zum binokularen Entfernungssehen der Insekten. 1973. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 1973; 87:165 - 88; http://dx.doi.org/10.1007/BF01352159