Abstract
The arbuscular mycorrhizal fungi (AMF) are important symbionts of land plants, which are known for their tremendous positive effects on terrestrial ecosystems, their peculiar cellular features, and their very old evolutionary history. To date, no sexual stage or apparatus have ever been observed in these organisms; a remarkable absence for a eukaryotic lineage. For this reason, AMF have long been considered an evolutionary oddity, having evolved for over 500 millions of years in the absence of sexual reproduction and meiosis. Here, we discuss the recent identification across a number of AMF genomes, of many genes that are known to be involved in the process of meiosis in several eukaryotic model species. The presence of these genes in AMF is a previously unsuspected and highly intriguing finding, which suggests the presence of a “hidden” sexual (or parasexual) reproduction that awaits formal observation in these poorly studied fungi.
Arbuscular mycorrhizal fungi (AMF) represent an ancient fungal lineage, with a fossil record dating back to the Ordovician (i.e., over 500 million years ago), and which evolved a close symbiotic association with the roots of most land plants (e.g., the mycorrhizal symbiosis).Citation1-Citation6 Their extreme longevity has intrigued scientists for some time, because members of this group are thought to have evolved for this long in the absence of sexual reproduction (i.e. exclusively clonally). Long-term clonal evolution is extremely rare among eukaryotes, and for this reason AMF have been long recognized to represent a peculiar lineage of “ancient asexuals.”Citation7,Citation8 This assumption, however has been mostly based on the absence of a recognizable sexual cycle in these organisms, and by a controversial hypothesis that suggest the presence of perpetual heterokayosis in some species (AMF cells are multinucleated);Citation9-Citation11 so it is still virtually possible that AMF may have a hidden sexual reproduction that has not yet been captured using the microscopy tools currently available.Citation12 In a recent study, we aimed to detect the presence of potential sexuality in AMF by searching their genomes for evidence of genes involved in the process of meiosis.Citation13 Meiosis is considered a hallmark of sexual reproduction in eukaryotes, which is essential to recombine different alleles across homologous chromosomes and protect the nuclear content from deleterious mutations.Citation14,Citation15
AMF Genomes Have the Tools to Undergo a Conventional Meiosis
Even though a battery of sequencing and bioinfomatics tools are now available to sequence the genome of many organisms, the acquisition of large-scale sequence data from AMF is still a relatively hard task to accomplish. This is because AMF are obligate symbionts of plants with relatively large genomes,Citation16 and which require tedious and time consuming cultivating techniques for propagation.Citation17 For some time, the combination of these features has hampered the acquisition of large amount of pure, contaminant-free, nucleic acids that are necessary to perform genome sequencing using the different next generation platform that are currently available (i.e. pyrosequencing).Citation18
Fortunately, Halary et al.Citation13have recently circumvented this problem by growing many AMF species under axenic conditions, allowing them to extract DNA in quantities large enough to perform deep genome sequencing procedures. Upon sequencing, the authors assembled relevant genome information, which they readily searched for evidence of meiosis. These searches used a set of genes (a total of 87) that are known to be involved at different stages of meiosis in the model eukaryote Saccharomyces cerevisiae, as queries against publicly available AMF transcriptome sequence data and newly acquired genome surveys from four species in the genus Glomus (i.e., Glomus irregulare, Glomus diaphanum, Glomus clarum and Glomus cerebriforme). These in silico approaches were complemented by degenerate PCR procedures, resulting in the identification of 51 AMF genes with strong homology with meiotic genes from other fungi. This gene set encodes for most of those proteins involved in the “core meiotic machinery”; a proteomic repertoire that is generally conserved across all sexual eukaryotes.Citation14,Citation15,Citation19 This gene catalog also included seven members whose products are only known to be involved in meiosis (and no other processes) in other eukaryotes (e.g. Dmc1, Rec8, Spo11, Mnd1, Hop2, Msh4 and Msh5); including other fungi. Importantly, the orthology of AMF meiosis-specific was confirmed using a variety of phylogenetic methods and gene homologs from many other organisms. The identification of a conserved and expanded catalog of meiotic genes in AMF, and the confirmation of their orthology, both suggest that a typical meiosis could occur in these supposedly ancient asexual fungi. If true, AMF may not be considered as an evolutionary aberration anymore, but rather as being similar to many other well studied sexual fungi that typically undergo meiosis following exchange of genetic material and nuclear fusion.
Meiosis Has Yet to be Observed in AMF
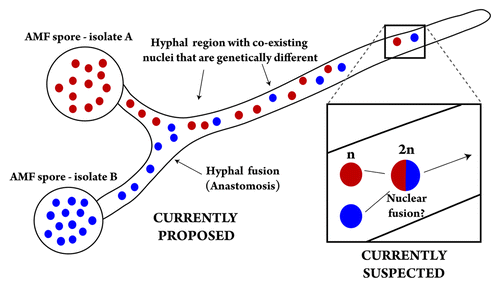
Obviously, many questions remain as to whether these newly identified genes are truly used for meiosis by AMF; especially because this process has so far never been observed in members of this fungal lineage. AMF nuclei are currently thought to be haploid, so one mechanism that could trigger meiosis in this group is nuclear fusion. In particular, similar cellular events are known to create diploid nuclei in other fungi, which readily undergo meiosis to produce a haploid nuclear progeny with novel allelic combinations ().Citation20-Citation22 This mechanism may also be followed by certain AMF individuals to reduce the load of deleterious mutations that are carried by their nuclei (e.g., Muller’s ratchet). Unfortunately, however, although nuclear exchange has been reported to occur between members of one AMF species following anastomoses (cytoplasmic fusion and exchange between hyphae),Citation9,Citation23,Citation24 nuclear fusions have yet to be observed in these fungi. Certainly, future studies aimed at detecting the presence of fusion events using, for instance, a variety of microscopy tools are certainly warranted. Specifically, these may reveal the mechanisms that are used by AMF to buffer deleterious mutations, and would shed much needed light on our understanding of their highly successful ecology and evolution by complementing previous work reporting the presence of recombination within some of their populations.Citation25-Citation27
Figure 1. Causes and “potential” consequences of nuclear exchange in the arbuscular mycorrhizal fungi (AMF). Schematic representation of cellular events that may occur between genetically different AMF individuals following anastomosis; based on reference Citation7 Genetically different nuclei are shown in different colors (red and blue). Meiosis is unknown to occur in AMF and nuclear has only been reported to occur between AMF individuals of one species (Glomus intraradices).
AMF May Not be that Shy After All
Several organisms long thought have evolved clonally have ultimately been found to undergo frequent sex,Citation8,Citation12,Citation19,Citation28-Citation33 and recent discoveries suggest that AMF may be no different.Citation13 Indeed, these supposedly ancient asexual fungi are now known to possess all the tools necessary to undergo a typical meiosis, and this specific phenomenon is likely to have caused the footprints of recombination that have been reported in a number of AMF populations.Citation25-Citation27 Hopefully, future studies will provide new insights into the presence, or absence, of sexual reproduction in these organisms; as these features may be used for the application of these obligate plant symbionts in organic agriculture and environmental practices. In parallel, AMF genomes should also be searched for other genes known to be involved in sexual reproduction and recognition in other fungi; including those that may compose a potential AMF mating type locus.Citation22
Acknowledgments
NC is a scholar of the Integrated Microbial Diversity program of the Canadian Institute for Advanced Research (CIFAR-IMB). Work in our lab is supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canadian fund for Innovation, the Ontario ministry of research and the RDP program from the University of Ottawa. We thank Stephane Aris-Brosou and Christiane Charest for useful comments on a previous version of this manuscript.
References
- Bonfante P, Genre A. Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis. Nat Commun 2010; 1:48; http://dx.doi.org/10.1038/ncomms1046; PMID: 20975705
- Bonfante P, Selosse MA. A glimpse into the past of land plants and of their mycorrhizal affairs: from fossils to evo-devo. New Phytol 2010; 186:267 - 70; http://dx.doi.org/10.1111/j.1469-8137.2010.03196.x; PMID: 20409182
- Humphreys CP, Franks PJ, Rees M, Bidartondo MI, Leake JR, Beerling DJ. Mutualistic mycorrhiza-like symbiosis in the most ancient group of land plants. Nat Commun 2010; 1:103; http://dx.doi.org/10.1038/ncomms1105; PMID: 2104582
- Rosendahl S. Communities, populations and individuals of arbuscular mycorrhizal fungi. New Phytol 2008; 178:253 - 66; http://dx.doi.org/10.1111/j.1469-8137.2008.02378.x; PMID: 18248587
- Sanders IR. Ecology and evolution of multigenomic arbuscular mycorrhizal fungi. Am Nat 2002; 160:Suppl 4 S128 - 41; http://dx.doi.org/10.1086/342085; PMID: 18707450
- Smith SE, Read DJ, Harley JL. Mycorrhizal symbiosis. San Diego, Calif.: Academic Press, 1997.
- Sanders IR, Croll D. Arbuscular mycorrhiza: the challenge to understand the genetics of the fungal partner. Annu Rev Genet 2010; 44:271 - 92; http://dx.doi.org/10.1146/annurev-genet-102108-134239; PMID: 20822441
- Smith JM. Evolution: contemplating life without sex. Nature 1986; 324:300 - 1; http://dx.doi.org/10.1038/324300a0; PMID: 3785401
- Angelard C, Sanders IR. Effect of segregation and genetic exchange on arbuscular mycorrhizal fungi in colonization of roots. New Phytol 2011; 189:652 - 7; http://dx.doi.org/10.1111/j.1469-8137.2010.03602.x; PMID: 21166810
- Hijri M, Sanders IR. Low gene copy number shows that arbuscular mycorrhizal fungi inherit genetically different nuclei. Nature 2005; 433:160 - 3; http://dx.doi.org/10.1038/nature03069; PMID: 15650740
- Kuhn G, Hijri M, Sanders IR. Evidence for the evolution of multiple genomes in arbuscular mycorrhizal fungi. Nature 2001; 414:745 - 8; http://dx.doi.org/10.1038/414745a; PMID: 11742398
- Kuck U, Poggler S. Cryptic sex in fungi. Fungal Biol Rev 2009; 23:86 - 90; http://dx.doi.org/10.1016/j.fbr.2009.10.004
- Halary S, Malik SB, Lildhar L, Slamovits CH, Hijri M, Corradi N. Conserved meiotic machinery in Glomus spp., a putatively ancient asexual fungal lineage. Genome Biol Evol 2011; 3:950 - 8; http://dx.doi.org/10.1093/gbe/evr089; PMID: 21876220
- Schurko AM, Logsdon JM Jr. Using a meiosis detection toolkit to investigate ancient asexual “scandals” and the evolution of sex. Bioessays 2008; 30:579 - 89; http://dx.doi.org/10.1002/bies.20764; PMID: 18478537
- Schurko AM, Neiman M, Logsdon JM Jr. Signs of sex: what we know and how we know it. Trends Ecol Evol 2009; 24:208 - 17; http://dx.doi.org/10.1016/j.tree.2008.11.010; PMID: 19282047
- Sędzielewska KA, Fuchs J, Temsch EM, Baronian K, Watzke R, Kunze G. Estimation of the Glomus intraradices nuclear DNA content. New Phytol 2011; 192:794 - 7; http://dx.doi.org/10.1111/j.1469-8137.2011.03937.x; PMID: 21988748
- Sędzielewska KA, Fuchs J, Temsch EM, Baronian K, Watzke R, Kunze G. Estimation of the Glomus intraradices nuclear DNA content. New Phytol 2011; 192:794 - 7; http://dx.doi.org/10.1111/j.1469-8137.2011.03937.x; PMID: 21988748
- Martin F, Gianinazzi-Pearson V, Hijri M, Lammers P, Requena N, Sanders IR, et al. The long hard road to a completed Glomus intraradices genome. New Phytol 2008; 180:747 - 50; http://dx.doi.org/10.1111/j.1469-8137.2008.02671.x; PMID: 19138232
- Malik SB, Pightling AW, Stefaniak LM, Schurko AM, Logsdon JM Jr. An expanded inventory of conserved meiotic genes provides evidence for sex in Trichomonas vaginalis. PLoS One 2008; 3:e2879; http://dx.doi.org/10.1371/journal.pone.0002879; PMID: 18663385
- Heitman J. Sexual reproduction and the evolution of microbial pathogens. Curr Biol 2006; 16:R711 - 25; http://dx.doi.org/10.1016/j.cub.2006.07.064; PMID: 16950098
- Heitman J. Evolution of eukaryotic microbial pathogens via covert sexual reproduction. Cell Host Microbe 2010; 8:86 - 99; http://dx.doi.org/10.1016/j.chom.2010.06.011; PMID: 20638645
- Lee SC, Ni M, Li WJ, Shertz C, Heitman J. The evolution of sex: a perspective from the fungal kingdom. Microbiol Mol Biol Rev 2010; 74:298 - 340; http://dx.doi.org/10.1128/MMBR.00005-10; PMID: 20508251
- Croll D, Giovannetti M, Koch AM, Sbrana C, Ehinger M, Lammers PJ, et al. Nonself vegetative fusion and genetic exchange in the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol 2009; 181:924 - 37; http://dx.doi.org/10.1111/j.1469-8137.2008.02726.x; PMID: 19140939
- Giovannetti M, Azzolini D, Citernesi AS. Anastomosis formation and nuclear and protoplasmic exchange in arbuscular mycorrhizal fungi. Appl Environ Microbiol 1999; 65:5571 - 5; PMID: 10584019
- Croll D, Sanders IR. Recombination in Glomus intraradices, a supposed ancient asexual arbuscular mycorrhizal fungus. BMC Evol Biol 2009; 9:13; http://dx.doi.org/10.1186/1471-2148-9-13; PMID: 19146661
- den Bakker HC, Vankuren NW, Morton JB, Pawlowska TE. Clonality and recombination in the life history of an asexual arbuscular mycorrhizal fungus. Mol Biol Evol 2010; 27:2474 - 86; http://dx.doi.org/10.1093/molbev/msq155; PMID: 20566475
- Vandenkoornhuyse P, Leyval C, Bonnin I. High genetic diversity in arbuscular mycorrhizal fungi: evidence for recombination events. Heredity (Edinb) 2001; 87:243 - 53; http://dx.doi.org/10.1046/j.1365-2540.2001.00941.x; PMID: 11703516
- Bennett RJ. Coming of age--sexual reproduction in Candida species. PLoS Pathog 2010; 6:e1001155; http://dx.doi.org/10.1371/journal.ppat.1001155; PMID: 21203475
- Dyer PS, O’Gorman CM. A fungal sexual revolution: Aspergillus and Penicillium show the way. Curr Opin Microbiol 2011; 14:649 - 54; http://dx.doi.org/10.1016/j.mib.2011.10.001; PMID: 22032932
- Judson OP, Normark BB. Ancient asexual scandals. Trends Ecol Evol 1996; 11:41 - 6; http://dx.doi.org/10.1016/0169-5347(96)81040-8; PMID: 21237759
- O’Gorman CM, Fuller HT, Dyer PS. Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature 2009; 457:471 - 4; http://dx.doi.org/10.1038/nature07528; PMID: 19043401
- Pöggeler S. Genomic evidence for mating abilities in the asexual pathogen Aspergillus fumigatus. Curr Genet 2002; 42:153 - 60; http://dx.doi.org/10.1007/s00294-002-0338-3; PMID: 12491009
- Scazzocchio C. Aspergillus genomes: secret sex and the secrets of sex. Trends Genet 2006; 22:521 - 5; http://dx.doi.org/10.1016/j.tig.2006.08.004; PMID: 16911845