Abstract
We have previously synthesized a caged form of the use-dependent N-methyl-D-aspartate (NMDA) receptor ion channel blocker MK801 and used intracellular photolysis of this compound to demonstrate the subcellular location of NMDA receptor ion channels involved in synaptic plasticity. Here, we discuss considerations regarding the choice of caging molecule, synthesis and the potential uses for caged ion channel blockers in neurophysiology.
Keywords: :
Introduction
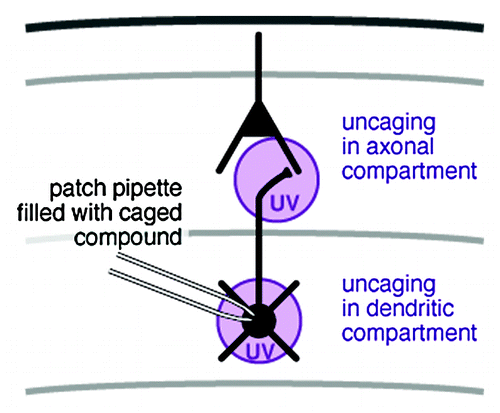
Pharmacological interventions are a valuable tool for the dissection of biological functions. In the study of excitable cells, such as neurons, drugs that block ion channels are of particular use. In acute brain slices, drug effects are commonly restricted to three levels: (1), the whole tissue preparation through addition of the drug to the incubation medium; (2), a small volume of tissue through local pressure injection or microiontophoresis; (3), a single cell through addition of the drug to the whole-cell patch-clamp pipette. Through photolysis of a novel caged NMDA receptor antagonist, added via a patch pipette, we recently demonstrated control of ion channel activity at the subcellular levelCitation1 (). This is of particular interest when studying the localization of ion channel function in highly compartmentalised cells such as neurons.
Cage Choice Considerations
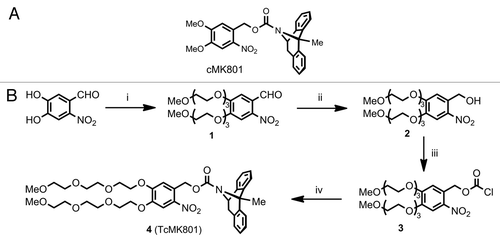
A number of photolabile compounds have been used in the past to achieve improved spatial and temporal control of free calcium, neurotransmitters, intracellular signaling molecules and ion channel blockers (for review, see ref. Citation2). We based our design on the nitrophenyl-based photolabile protecting group (cage) nitroveratryloxycarbonyl (NVOC) rather than other commonly employed cages such as methoxy nitroindolinyl (MNI)Citation3 or nitrophenyl ethyl (NPE)Citation4 since it could be easily derivatised at its phenolic positions. Due to the relatively low uncaging efficiency of the NVOC group, it may be handled under ambient light conditions and excellent contrast can be achieved between subcellular compartments. NVOC cages are neuropharmocologically compatible; we showed that the cage itself does not cause any channel blocking, unlike MNI which interacts with GABAA receptors.Citation5 We synthesized both NVOC-protected MK801 (cMK801, ) and a novel triethylene glycol-substituted nitrophenyl cage-protected MK801 (TcMK801, ). Formulation of cMK801 with Polysorbate 20 afforded sufficient water-solubility, though was detrimental to quick patch-clamp seal formation. Water solubility conferred instead by the triethylene glycol chains of TcMK801 permitted a formulation-free solution of caged MK801, which permits seal formation, as with standard aqueous intracellular solutions.
Figure 2. (A) Nitroveratryloxycarbonyl-protected MK801 (cMK801). (B) Synthesis of TEG-substituted caged MK801 (TcMK801) from 4,5-dihydroxy-2-nitrobenzaldehyde. (1) Triethylene glycol monomethyl monotosylate ether, K2CO3, MeCN, reflux, 16 h. (2) NaBH4, MeOH, rt, 3 h. (3) (Cl3CO)2CO, Et3N, CHCl3, rt, 2.5 h. (4) Dizocilpine maleate (MK801), Na2CO3, dioxane, THF, water, rt, 48 h.
Compound Synthesis and Improvement of Water-Solubility
4,5-Bis{2-[2-(2-methoxyethoxy)ethoxy]ethoxy}-2-nitrobenzaldehyde
This compound was prepared according to an adapted literature procedure.Citation6 A solution of 4,5-dihydroxy-2-nitrobenzaldehyde (60 mg, 327 μmol), 2-[2-(2-methoxyethoxy)ethoxy]ethyl tosylate (209 mg, 655 μmol) and K2CO3 (90 mg, 655 μmol) in anhydrous acetonitrile (5 mL) was refluxed for 16 h under N2. The mixture was evaporated to dryness and purified by flash chromatography (ethyl acetate). The combined fractions were evaporated to dryness to yield 1 as a yellow oil (92 mg, 59%). 1H NMR (400 MHz, CDCl3) δH 10.43 (s, 1H), 7.71 (s, 1H), 7.45 (s, 1H), 4.32 (m, 4H), 3.94 (m, 4H), 3.75 (s, 4H), 3.66 (m, 8H), 3.55 (m, 4H), 3.38 (s, 6H). 13C NMR (100 MHz, CDCl3) δC 188.16, 153.39, 152.23, 144.19, 125.99, 111.87, 109.61, 72.35, 71.43, 71.13, 71.03, 69.91, 69.71, 59.48. m/z (ESI+) 498.18 ([M+Na]+ 100%, C21H33ClNNaO11+ requires 498.20).
|4,5-Bis{2-[2-(2-methoxyethoxy)ethoxy]ethoxy}-2-nitrophenyl|methanol
This compound was prepared according to an adapted literature procedure.Citation6 NaBH4 (18.5 mg, 490 μmol) was added portionwise to a solution of 4,5-bis{2-[2-(2-methoxyethoxy)ethoxy]ethoxy}-2-nitrobenzaldehyde 1 (46.6 mg, 98 μmol) in anhydrous methanol (1.5 mL) at 5°C. The reaction mixture was allowed to warm to room temperature and stirred for 3 h. The reaction was quenched with HCl(aq) (5 mL, 1.0 M) and extracted with chloroform (3 × 10 mL). The combined organic layers were washed with saturated NaCl solution (5 mL) and evaporated to dryness to yield 2 as a colorless oil (30 mg, 64%). 1H NMR (400 MHz, CDCl3) δH 7.76 (s, 1H), 7.35 (s, 1H), 4.95 (s, 2H), 4.33 (m, 2H), 4.23 (m, 2H), 3.90 (m, 4H), 3.74 (m, 4H), 3.66 (m, 8H), 3.55 (m, 4H), 3.37 (s, 3H), 3.36 (s, 3H), 1.25 (s, 1H). 13C NMR (100 MHz, CDCl3) δC 153.86, 147.25, 139.44, 133.19, 112.74, 110.56, 71.90, 71.87, 70.90, 70.79, 70.69, 70.65, 70.55, 70.43, 69.78, 69.51, 69.14, 68.89, 62.50, 59.01. m/z (ESI+) 500.16 [(M+Na)+ 100%, C21H35NNaO11+ requires 500.21].
4,5-Bis{2-[2-(2-methoxyethoxy)ethoxy]ethoxy}-2-nitrobenzyl chloroformate
A mixture of |4,5-bis{2-[2-(2-methoxyethoxy)ethoxy]ethoxy}-2-nitrophenyl|methanol 2 (30 mg, 63 μmol) and triphosgene (28 mg, 94.5 μmol) in chloroform (5 mL) was stirred for 2 h. Further triphosgene (18 mg, 63 μmol) was added and the mixture stirred for 30 min. When the reaction was complete by thin layer chromatography (TLC), the mixture was evaporated to dryness and purified by column chromatography on SiO2 (1% MeOH in ethyl acetate). Combined fractions were evaporated and dried in vacuo to yield the product 3 as a colorless oil (20 mg, 59%). 1H NMR (400 MHz, CDCl3) δH 7.83 (s, 1H), 7.06 (s, 1H), 5.70 (s, 2H), 4.26 (m, 4H), 3.93 (m, 4H), 3.73 (m, 4H), 3.67 (m, 8H), 3.57 (m, 4H), 3.38 (s, 6H). m/z (ESI+) 562.18 [(M+Na)+ 100%, C22H34ClNNaO12+ requires 562.16]
TcMK801
This compound was prepared according to an adapted literature procedure.Citation7 A solution of 4,5-bis{2-[2-(2-methoxyethoxy)ethoxy]ethoxy}-2-nitrobenzyl chloroformate 3 (20 mg, 37 μmol) in tetrahydrofuran (THF, 0.5 mL) was added to a solution of (+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate (MK801, 12.4 mg, 37 μmol), Na2CO3 monohydrate (18.4 mg, 148 μmol) and dioxane (0.5 mL) in water (1.0 mL). The mixture was stirred for 48 h. The reaction mixture was poured onto saturated Na2CO3(aq) (2 mL), extracted with chloroform (2 × 5 mL) and washed with water (2 × 5 mL). The organic layer was evaporated to dryness and purified by column chromatography on SiO2 (1% MeOH in ethyl acetate). Combined fractions were evaporated and dried in vacuo to yield the product 4 as a colorless oil (8 mg, 29%). 1H NMR (400 MHz, CDCl3) δH 7.75 (m, 1H), 7.33 (m, 1H), 7.31 (m, 2H), 7.19 (m, 2H), 7.10 (m, 2H), 7.08 (m, 1H), 6.92 (m, 1H), 5.51 (s, 1H), 5.49 (s, 1H), 4.22 (m, 2H), 3.88 (m, 2H), 3.82 (m, 4H), 3.74 (m, 4H), 3.65 (m, 8H), 3.55 (m, 4H), 3.38 (s, 3H), 3.37 (s, 3H), 2.69 (d, J = 17.1 Hz, 1H), 2.26 (s, 3H), 1.26 (s, 2H). 13C NMR (100 MHz, CDCl3) δC 207.00, 167.75, 153.33, 143.27, 139.16, 130.86, 130.44, 128.78, 127.64, 127.52, 127.41, 126.13, 121.28, 118.60, 110.79, 71.90, 70.91, 70.66, 70.54, 69.47, 69.21, 68.79, 67.95, 67.06, 59.86, 59.01, 38.70, 30.91, 30.33, 29.67, 28.89, 25.58, 23.72, 22.96, 22.67, 14.03, 10.94. m/z (ESI+) 747.3100 [(M+Na)+ 100%, C38H48N2NaO12+ requires 747.3099].
Potential Uses
A wide variety of caging groups exists, making it possible to tailor the photolysis of a caged compound to suit desired experimental conditions. Judicious choice of caging group allows specification of photolysis wavelength, temporal release behavior and light sensitivity. Photolysis can be achieved with millisecond precision, enabling the study of fast events like calciumCitation8,Citation9 and neurotransmitterCitation10-Citation12 signaling and slower events such as the expression of synaptic plasticity.Citation1
The caged compounds cMK801 and TcMK0801 presented here are effective under one-photon activation. Greater spatial selectivity and depth penetration may be achieved by two-photon excitation due to the reduction in scattering and excitation out of the focal plane. Advances in two-photon photolysisCitation8 promise to improve spatial resolution and allow the precisely defined uncaging of compounds in volumes as small as 1 µm3. A two-photon-optimized caged potassium channel blocker is commercially availableCitation13 and we are directing efforts toward preparing two-photon-optimized variants of our caged NMDA receptor channel blocker. Using these compounds to selectively block neuronal conductances via two-photon photolysis will enable the dissection of neuronal signal integration in dendritic sub-compartments. This approach can be extended to other pharmacologically active substances, allowing the study of cellular function and signaling at unprecedented levels of detail.
Acknowledgments
This work was supported by the Biotechnology and Biological Sciences Research Council, UK and the Wellcome Trust OXION initiative. A.R.M. was supported by a short-term fellowship from the Alicia Koplowitz Foundation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Rodríguez-Moreno A, Kohl MM, Reeve JE, Eaton TR, Collins HA, Anderson HL, et al. Presynaptic induction and expression of timing-dependent long-term depression demonstrated by compartment-specific photorelease of a use-dependent NMDA receptor antagonist. J Neurosci 2011; 31:8564 - 9; http://dx.doi.org/10.1523/JNEUROSCI.0274-11.2011; PMID: 21653860
- Ellis-Davies GCR. Caged compounds: photorelease technology for control of cellular chemistry and physiology. Nat Methods 2007; 4:619 - 28; http://dx.doi.org/10.1038/nmeth1072; PMID: 17664946
- Canepari M, Nelson L, Papageorgiou G, Corrie JET, Ogden D. Photochemical and pharmacological evaluation of 7-nitroindolinyl-and 4-methoxy-7-nitroindolinyl-amino acids as novel, fast caged neurotransmitters. J Neurosci Methods 2001; 112:29 - 42; http://dx.doi.org/10.1016/S0165-0270(01)00451-4; PMID: 11640955
- Kaplan JH, Forbush B 3rd, Hoffman JF. Rapid photolytic release of adenosine 5′-triphosphate from a protected analogue: utilization by the Na:K pump of human red blood cell ghosts. Biochemistry 1978; 17:1929 - 35; http://dx.doi.org/10.1021/bi00603a020; PMID: 148906
- Fino E, Araya R, Peterka DS, Salierno M, Etchenique R, Yuste R. RuBi-Glutamate: two-photon and visible-light photoactivation of neurons and dendritic spines. Front Neural Circuits 2009; 3:1 - 9; http://dx.doi.org/10.3389/neuro.04.002.2009; PMID: 19225575
- Shin A, Yoshinari O, Masami O, Kazuo U. Synthesis of the indole core structures of conophylline. J Heterocycl Chem 2008; 45:1803 - 8; http://dx.doi.org/10.1002/jhet.5570450638
- Steward LE, Collins CS, Gilmore MA, Carlson JE, Ross JBA, Chamberlin AR. In vitro site-specific incorporation of fluorescent probes into β-galactosidase. J Am Chem Soc 1997; 119:6 - 11; http://dx.doi.org/10.1021/ja963023f
- Adams SR, Kao JPY, Grynkiewicz G, Minta A, Tsien RY. Biologically useful chelators that release Ca2+ upon illumination. J Am Chem Soc 1988; 110:3212 - 20; http://dx.doi.org/10.1021/ja00218a034
- Ellis-Davies GCR, Kaplan JH. Nitrophenyl-EGTA, a photolabile chelator that selectively binds Ca2+ with high affinity and releases it rapidly upon photolysis. Proc Natl Acad Sci U S A 1994; 91:187 - 91; http://dx.doi.org/10.1073/pnas.91.1.187; PMID: 8278362
- Walker JW, McCray JA, Hess GP. Photolabile protecting groups for an acetylcholine receptor ligand. Synthesis and photochemistry of a new class of o-nitrobenzyl derivatives and their effects on receptor function. Biochemistry 1986; 25:1799 - 805; http://dx.doi.org/10.1021/bi00355a052; PMID: 3707910
- Wieboldt R, Gee KR, Niu L, Ramesh D, Carpenter BK, Hess GP. Photolabile precursors of glutamate: synthesis, photochemical properties, and activation of glutamate receptors on a microsecond time scale. Proc Natl Acad Sci U S A 1994; 91:8752 - 6; http://dx.doi.org/10.1073/pnas.91.19.8752; PMID: 8090718
- Matsuzaki M, Ellis-Davies GCR, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci 2001; 4:1086 - 92; http://dx.doi.org/10.1038/nn736; PMID: 11687814
- Nikolenko V, Yuste R, Zayat L, Baraldo LM, Etchenique R. Two-photon uncaging of neurochemicals using inorganic metal complexes. Chem Commun (Camb) 2005; 13:1752 - 4; http://dx.doi.org/10.1039/b418572b; PMID: 15791321