Abstract
Pupae of some insects produce sounds or vibrations, but the function of the sounds/vibrations has not been clarified in most cases. Recently, we found vibratory communication between pupae and larvae of a group-living beetle Trypoxylus dichotoma, which live in humus soil. The vibratory signals produced by pupae were shown to deter approaching larvae, thereby protecting themselves. In the present study, we tested our hypothesis that pupal signals are mimics of vibratory noises associated with foraging of moles, the most common predators of T. dichotoma. Mole vibrations played back in laboratory experiments deterred larval approaches in the same way as pupal signals. These findings suggest that to deter conspecific larvae, pupae of T. dichotoma may have exploited a preexisting response of larvae to predator vibrations by emitting deceptive signals.
Insect pupae are generally considered inactive and quiescent, but some of them generate air-borne sounds and/or substrate-borne vibrations.Citation1-Citation4 In 1948, Hinton reviewed the mechanisms of sound/vibration production in the pupae of Lepidoptera,Citation2 and suggested a defensive function against predators. Since then, the functions of pupal and larval vibrations/sounds have been examined intensively in ant-associated lycaenid butterflies. Larvae of the common imperial blue Jalmenus evagoras offer nutritious secretions to Myrmicinae ants, and in return the ants protect larvae and pupae of this butterfly from parasitoids and other enemies. The larvae and pupae of J. evagoras generate vibrations, which induce higher levels of ant attendance.Citation3 Likewise, sounds produced by larvae and pupae of the mountain Alcon blue Maculinea rebeli mimic the sounds produced by queen ants, which can elicit additional attention and favor by worker ants.Citation4 To our knowledge, the function of pupal sounds/vibrations in other groups of insects has remained unexplored.
Recently, we revealed a deterring function of pupal vibrations against conspecific larvae in a group-living Japanese rhinoceros beetle Trypoxylus dichotoma (Coleoptera, Scarabaeidae, Dynastinae).Citation5 They construct their own oval pupal cells around them by compacting a mixture of fecal pellets and humus to secure normal pupation and eclosion (). However, the pupal cells are so fragile that they can be broken by even slight disturbances. The pupal cells are at high risk of being accidentally damaged by the burrowing conspecific larvae because pupae and larvae live close together ().Citation5 In a previous paper, we reported that pupae emitted vibrations () by drumming their pronotum on the inner wall of pupal cells in response to approaching larvae.Citation5 We tested if the pupal vibrations function as deterring signals to larvae. Pupal cells harboring a live pupa were less likely to be broken by larvae than those harboring a dead pupa.Citation5 When pupal vibrations were played back near to vacant artificial pupal cells, these cells were rarely disturbed by the larvae.Citation5 These results suggest that pupae generate vibrations to deter conspecific larvae, thereby preventing damage to the cells.
Figure 1. Pupae and pupal cells of two Scarabaeidae species. (A) A male pupa of Trypoxylus dichotoma (Dynastinae) with a long head horn residing in a fragile cell. (B) A pupa of Dicronorhina derbyana (Cetoniinae) residing in a hard cell. Note that humus surrounding the cells was removed. Scale bar = 1 cm.
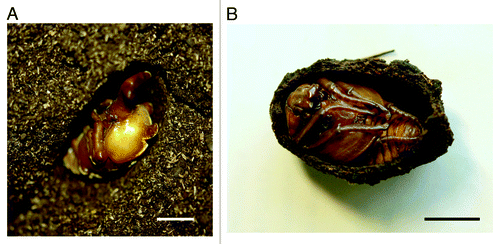
Figure 2. Interaction between pupae and larvae of Trypoxylus dichotoma. (A) A pupa in a pupal cell and a larva. The average distance between pupal cells and larvae was 6.4 cm in their natural habitat. (B) Oscillograms of vibrations produced by a male pupa in a pupal cell (upper) and vibrations produced by a foraging mole recorded by KC Catania (lower).Citation6 Arrows indicate the range used for playback experiments. (C) Proportion of larvae that broke artificial pupal cell(s) when pupal vibrations, background noise, or mole vibrations were played back. Sample sizes are shown in parentheses. NS: Not significant. ***p < 0.0003 by χ2 test with Bonferonni correction. (upper) are reproduced with kind permission of Springer Science+Business Media.
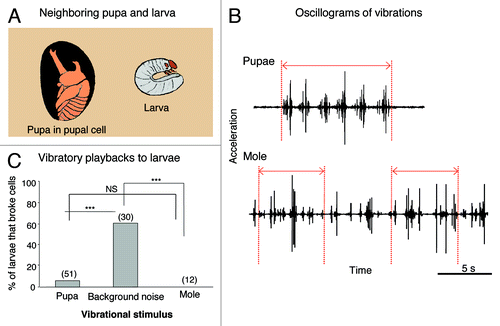
In the present study, we hypothesized that the larval response to pupal signals is derived from stereotyped anti-predatory responses such as startle and freezing, which are induced upon detecting vibratory noises produced by predators. If this is true, pupal signals are mimics of predator noises. We therefore examined if larvae are deterred by the predator noises. We chose moles (Mogera imaizumi) as common predators of T. dichotoma in the field. In 27% (3/11) of microhabitats examined (Tamakyuryo Hill), the marks of mole’s feeding on T. dichotoma larvae were found, and in these microhabitats, few surviving larvae were found. Vibratory noises produced in association with moles’ foraging have been recently characterized.Citation6 The mole vibrations and pupal signals are similar in that both predominantly contain low-frequency components (mostly below 500 Hz) and successions of pulses at intervals of a few seconds (). When the mole vibrations were played back near to vacant artificial pupal cells (see Methods), these cells were never disturbed by the larvae (). This result clearly shows the deterring effect of mole vibrations on larvae. Pupae may have exploited a preexisting larval response to predatory noises by emitting deceptive signals for their own benefit. The larval response is likely to increase the chance of their survival, considering that various fossorial mammals detect prey using vibrations associated with their activity.Citation7 Larvae probably incur a fitness cost as well by responding to ‘false’ predatory cues from pupae (e.g., restriction of activity). However, given that adult females lay dozens of eggs at one siteCitation8 and sibs live close together, the cost may be partially compensated for by avoiding the ‘killing’ of sibs (i.e., increased inclusive fitness).
How have pupal signals evolved in T. dichotoma? Pupae of some other coleopteran species such as ladybird beetles and mealworms are reported to show behavioral defenses such as abdominal rotation and intersegmental pinching behavior.Citation9,Citation10 When pupae of T. dichotoma produce vibratory signals, drumming of the cell wall is achieved by rotating their abdomen.Citation5 Therefore, it is possible that production of vibratory signals in T. dichotoma evolved from defensive behaviors against predators under the selective force imposed by conspecific larvae. The fragility of pupal cells may also be associated with the evolution of pupal signals in this species. We examined the abdominal rotation behavior in pupae of Cetoniinae species (e.g., Protaetia orientalis, Pseudotorynorhina japonica and Dicronorhina derbyana), which belong to the same Scarabeidae as T. dichotoma (Dynastinae). Like T. dichotoma, the larvae of Cetoniinae species feed on humus and show a highly clumped distribution. However, in contrast to T. dichotoma, the pupae of Cetoniinae species construct a very hard pupal cell from fecal pellets and humus (). We found that they rarely show abdominal rotation in response to contact or vibratory stimuli. It is probable that vibratory defense is unnecessary for Cetoniinae pupae because the hard cells protect them from disturbances. Thus, vibratory communication in T. dichotoma may have evolved along with some anti-predation characteristics, such as defensive behavior and structure of pupal cells.
Methods
For playback experiments, we used a previously described experimental system,Citation5 where vibrations were played back near to vacant artificial pupal cells. In brief, a plastic container (30 cm × 7 cm × 7 cm) was filled with humus soil and two artificial cells were prepared by briefly thrusting a test tube (3 cm in diam.) into the soil. The container was suspended with a plastic string from the ceiling of a one-side-opened soundproof box. The tips of a U-shaped gadget connected to a vibration exciter (type 4809, Brüel and Kjær) were placed under the artificial cells through holes made at the bottom of the container. For pupal vibrations, we clipped a sequence (ca. 4.5–7 sec) of vibrations containing 3–7 pulses.Citation5 For mole vibrations, we clipped two sequences of vibrations containing several pulses (ca. 5 sec in total) from a recording file provided in reference Citation6 (). The vibrations were originally obtained from a field-collected mole (Scalopus aquaticus) by using a geophone and contained a broad frequency range with a peak near 200 Hz.Citation6 For controls, the background noises recorded along with the pupal vibrations was used. A larva was placed at the center of the container, and the vibrations were repeatedly played back at intervals of 10 sec for 60 min. The acceleration of played vibrations was 0.25 m/s2 at 10.5 cm from the vibratory source, nearly equal to that of natural pupal vibrations. At the end of trials, it was checked whether a larva in the humus had broken artificial pupal cells.
Acknowledgments
We thank Dr. Kenneth C. Catania for audio files used in our playback experiments. We also wish to thank Dr. Frantisek Baluska and the two anonymous referees for invaluable comments on the manuscript. Permission for use of figures from Springer Science+Business Media is acknowledged.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Hinton HE. The “gin-traps” of some beetle pupae; a protective device which appears to be unknown. Trans R Entomol Soc Lond 1946; 97:473 - 96; http://dx.doi.org/10.1111/j.1365-2311.1946.tb00273.x
- Hinton HE. Sound production in Lepidopterous pupae. Entomologist 1948; 81:254 - 69
- Travassos MA, Pierce NE. Acoustics, context and function of vibrational signalling in a lycaenid butterfly-ant mutualism. Anim Behav 2000; 60:13 - 26; http://dx.doi.org/10.1006/anbe.1999.1364; PMID: 10924199
- Barbero F, Thomas JA, Bonelli S, Balletto E, Schönrogge K. Queen ants make distinctive sounds that are mimicked by a butterfly social parasite. Science 2009; 323:782 - 5; http://dx.doi.org/10.1126/science.1163583; PMID: 19197065
- Kojima W, Takanashi T, Ishikawa Y. Vibratory communication in the soil: pupal signals deter larval intrusion in a group-living beetle Trypoxylus dichotoma. Behav Ecol Sociobiol 2012; 66:171 - 9; http://dx.doi.org/10.1007/s00265-011-1264-5
- Catania KC. Worm grunting, fiddling, and charming--humans unknowingly mimic a predator to harvest bait. PLoS One 2008; 3:e3472; http://dx.doi.org/10.1371/journal.pone.0003472; PMID: 18852902
- Mason MJ, Narins PM. Seismic signal use by fossorial mammals. Am Zool 2001; 41:1171 - 84; http://dx.doi.org/10.1668/0003-1569(2001)041[1171:SSUBFM]2.0.CO;2
- Tsurumaki H. Collecting and breeding of the Japanese rhinoceros beetle. [in Japanese] Saishu To Shiiku 1987; 49:254 - 7
- Eisner T, Eisner M. Operation and defensive role of “gin traps” in a coccinellid pupa (Cycloneda sanguinea). Psyche (Stuttg) 1992; 99:265 - 73
- Ichikawa T, Kurauchi T. Larval cannibalism and pupal defense against cannibalism in two species of tenebrionid beetles. Zoolog Sci 2009; 26:525 - 9; http://dx.doi.org/10.2108/zsj.26.525; PMID: 19719403