Abstract
We discuss potential chemical substances responsible for attracting acellular slime mold Physarun polycephalum to valerian root. The contributes toward fundamental research into pheromones and chemo-attracts of primitive organisms such as slime molds. The results show that significant information could be gained about the action of compounds on higher organisms.
Keywords: :
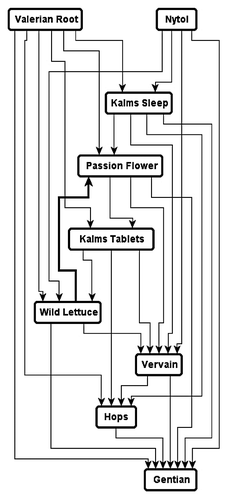
A plasmodium, the vegetative phase of the acellular slime mold Physarum polycephalum, has recently became a popular biological substrate for making experimental laboratory prototypes of living computing devices.Citation1 The Physarum-based computing devices are programmed using gradients fields generated by discrete configurations of chemo-attractants. Laboratory experiments shown that the plasmodium of P. polycephalum is attracted to glucose, maltose, mannose, galactose, many aminoacids (e.g., phenylalanine, leucine, serine, asparagine, theonine). Recently we found,Citation2 that the plasmodium is strongly attracted to herbal calming/somnipherous tablets NytolCitation3 and Kalms Sleep.Citation4 To select the principle chemo-attractant in the tablets we undertook laboratory experiments on the plasmodium's binary choice between samples of dried herbs/roots: Valeriana officinalis, Humulus lupulus, Passiflora incarnate, Lactuca virosa, Gentiana lutea, Verbena officinalis (). We constructed a hierarchy of chemo-attractive force () and found that Valerian root was the strongest chemo-attractant for P. polycephalum.Citation2 A possible link between sedative activity of valerian and its chemo-attraction—via relaxation of contractile activities—is outlined in our original paper.Citation2
Figure 1. An exemplar experimental setup. The plasmodium is inoculate in the center of a Petri dish and two portions of substances (Valerian root on the left and catnip on the right) and are placed at the end of diameter segment. See details in Citationreference 1.
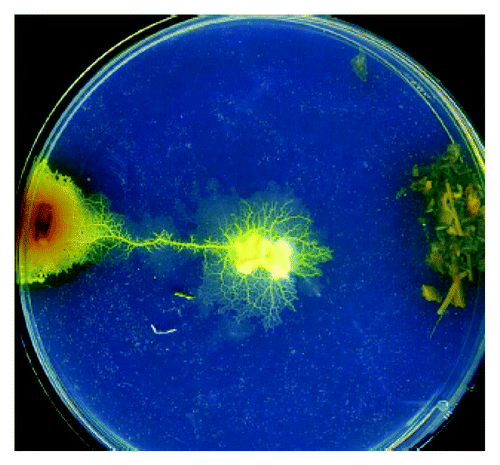
Figure 2. Hierarchy of Physarum preferences. The higher is a substance positioned in the hierarchy the more strongly the substance attracts P. polycephalum. From Citationreference 1.
Valerian contains hundreds of identified, and possibly the same amount of not yet identified, components including alkaloids, volatile oils, valerinol and actinidine ().Citation5-Citation8 We can postulate that slime mold in the plasmodium stage may be attracted to a plant because the plant roots or stem harbour high level of food (bacteria), or the plant may provide protection for the slime mold from insect predators (e.g., fungus gnats, round fungus beetle, many plants including Nepeta cataria are known to have insecticidal secretions), the plant's volatile secretions “pheromones” may be chemically similar to “pheromones” of slime mold. This may be coincidence or there may be some beneficial symbiosis between certain plants and slime molds (An example is the case of the recently discovered night flowering orchid which scientists believe mimics the plasmodial stage of a slime mold visually (chemically?) in order to attract pollinators.)
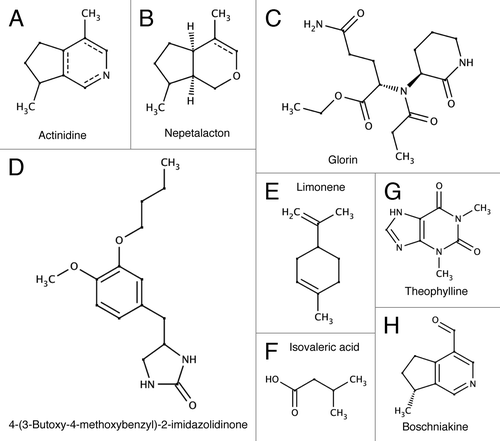
Actinidine is structurally close to the terpenoid nepetalactone (), the active substance of catnip Nepeta cataria. Nepetalactone and actinidine both have a similar bicyclic structural skeleton, and are classed as monoterpene derivatives. Actinidine as well as having the same dramatic attractive effect as Nepetalactone on cats, rats etc. is also a pheromone or allomone of many insect species (ant, stick insect). Boschniakine () also acts as a defense substance for stick insects,Citation9 and shares significant chemical structure with actinidine. So we may expect Boschniakine to impart a chemoattractive effect on Slime molds if the cyclic functionality is important for this action. It should be noted that in our experiments catnip exhibited a lesser slime mold attractive potential then valerian,Citation2 thus we can postulate that despite their structural similarities actinidine and nepetalactone act differently on the slime mold's metabolism. However, it is interesting that the two substances show such a range of activity across many species.
Isovaleric acid () and actinidine () are identified in the anal gland secretion of Iridomyrmex nitidiceps ant, and isovaleric acid is considered to be a distress indicator.Citation10 We can speculate that these components are also pheromones of P. polycephalum and can be considered in a framework of pheromones of cellular slime moldsCitation11-Citation13 (indeed, there may be pitfalls in projecting physiology of cellular mold to their acellular counterparts).
The slime molds are fairly primitive organisms. Therefore, we could argue that receptor bound by actinidine is a generic one and not developed to impart selectivity to one specific chemical.
Kincaid and MansourCitation14 found that inhibitors of the enzyme cyclic 3′,5′-AMP- phosphodiesterase act as strong or moderate chemoattractants in P. polycephalum. Among substances tested strongest effect was observed with 4-(-3-butoxy-4-methoxybenzyl)-2-imidazolidinone () and moderate effects from theophylline () and other xanthine derivatives (interestingly they observed negative chemotaxis at high concentrations). Theopylline () is quite similar to caffeine and has a similar chemical structure to actinidine (). They are bicyclic alkaloid/terpenoid molecules although the functionaliszation is distinct. Conversely, Nepetalactone does () not share the same structural similarities to the xanthine derivatives as actinidine. This may be the reason for the observed lesser effect as a chemoattractant for the slime mold P. polycephalum.
Acrasins (like cAMP), e.g., glorinCitation15 (), which are implicated in the aggregation of slime molds (not specifically physarum) also have certain structural similarities to compounds found in valerian. Limonene () and other terpenes have been found to bind to A2A adenosine receptors.Citation16 Other antagonists are caffeine, theophylline (), istradefylline. So molecules with very limited structural similarity can bind to major receptors and impart a range of metabolic effects on various species.
In conclusion even though the chemical structures of actinidine and nepetalacton are quite different, they induce the same behavior in cats, rats and act as strong or moderate attractants for slime molds. Thus we can postulate that the receptors involved are very non-specific and may have shared structure between primitive organisms and higher organisms. Therefore, there is significant “crosstalk” between pheromone like molecules and mimics—it appears especially when molecules have cyclic structure.
The original paper although searching for chemoattractants for applied research highlights the need for fundamental research into pheromones and chemo-attracts of primitive organisms such as slime molds. The results show that significant information could be gained about the action of compounds on higher organisms.
References
- Adamatzky A. Physarum Machines. Singapore: World Scientific, 2010.
- Adamatzky A. On attraction of slime mould Physarum polycephalum to plants with sedative properties. Nature Proc 2011; http://dx.doi.org/10.1038/npre.2011.5985.1
- GlaxoSmithKlin. 2011. Nytol leaflet. Swadlincot, Debyshire.
- G.R. Lane HealthProducts. 2011. Kalms Sleep leaflet. Gloucester, UK.
- Torssell K, Wahlberg K. The structure of the principal alkaloid from valeriana officinalis (L.). Tetrahedron Lett 1966; 7:445 - 8; http://dx.doi.org/10.1016/S0040-4039(00)72961-3
- Hendriks H, Bruins AP. Study of three types of essential oil of S1 by combined gas chromatography-negative ion chemical ionization mass spectrometry. J Chromatogr A 1980; 190:321 - 30; http://dx.doi.org/10.1016/S0021-9673(00)88236-9
- Jommi G, Krepinsky J, Herout V, Sorm F. The structure of valerianol, a sesquiterpenic alcohol of eremophilane type from valerianan oil. Tetrahedron Lett 1967; 8:677 - 81; http://dx.doi.org/10.1016/S0040-4039(00)90572-0
- Johnson RD, Wallera GR. Isolation of actinidine from valeriana officinalis. Phytochemistry 1971; 10:3334 - 5; http://dx.doi.org/10.1016/S0031-9422(00)97426-0
- Ho H-Y, Chow YS. Chemical identification of defensive secretion of stick insect, Megacrania tsudai Shiraki. J Chem Ecol 1990; 19:39 - 46; http://dx.doi.org/10.1007/BF00987469
- Cavill GWK, Robertson PL, Brophy JJ, Clark DV. Defensive and other secretions of the australian cocktail ant, Iridomyrmex Nitidiceps. Tetrahedron 1982; 38:1931 - 8; http://dx.doi.org/10.1016/0040-4020(82)80042-2
- Lewis KE, O’Day DH. Evidence for a hierarchical mating system operating via pheromones in Dictyostelium giganteum. J Bacteriol 1979; 138:251 - 3; PMID: 571432
- Newell PC. Chemotaxis in the cellular slime moulds. In: Biology of the Chemotactic Response. Lackie JM, Wilkinson PC (ed), Cambridge: Cambridge University Press, 1981.
- Nader WF, Shipley GL, Huettermann A, Holt CE. Analysis of an inducer of the amoebal-plasmodial transition in the myxomycetes Didymium iridis and Physarum polycephalum. Dev Biol 1984; 103:504 - 10; http://dx.doi.org/10.1016/0012-1606(84)90337-3; PMID: 6539260
- Kincaid RL, Mansour TE. Cyclic 3′,5′-AMP phosphodiesterase in Physarum polycephalum. I. Chemotaxis toward inhibitors and cyclic nucleotides. Biochim Biophys Acta 1979; 588:332 - 41; http://dx.doi.org/10.1016/0304-4165(79)90341-6; PMID: 228761
- Asghar A, Groth M, Siol O, Gaube F, Enzensperger C, Glöckner G, et al. Developmental gene regulation by an ancient intercellular communication system in social amoebae. Protist 2012; 163:25 - 37; http://dx.doi.org/10.1016/j.protis.2010.12.002; PMID: 21371934
- Park HM, Lee JH, Yaoyao J, Jun HJ, Lee SJ. Limonene, a natural cyclic terpene, is an agonistic ligand for adenosine A(2A) receptors. Biochem Biophys Res Commun 2011; 404:345 - 8; http://dx.doi.org/10.1016/j.bbrc.2010.11.121; PMID: 21134357