Abstract
The unfolded protein response (UPR) is an intracellular signaling pathway that regulates the cellular response to the accumulation of misfolded proteins in eukaryotes. Our group has demonstrated that cell wall stress activates UPR in yeast through signals transmitted by the cell wall integrity (CWI) mitogen-activated protein (MAP) kinase cascade. The UPR is required to maintain cell wall integrity; mutants lacking a functional UPR have defects in cell wall biosynthesis and are hypersensitive to cell wall-directed antifungal drugs. Since ER stress also activates CWI signaling, we propose that ER and cell wall stress responses are coordinated by CWI and UPR signaling pathways in order to protect cells against these related stressors. Further investigation of the mechanisms of this coordinate regulation may lead to improved cell wall-directed antifungal therapies.
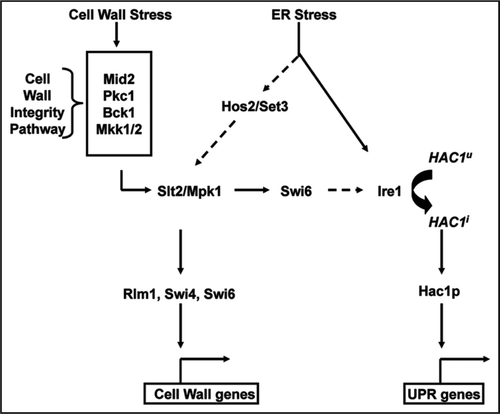
The unfolded protein response (UPR) is an intracellular signaling pathway that is activated principally by the accumulation of misfolded proteins in the endoplasmic reticulum (ER) of eukaryotic cells.Citation1 In metazoan cells, UPR is regulated by three transducers (Ire1, PERK and ATF6) that function in an inter-related fashion.Citation2 In yeast, UPR is controlled only by Ire1p through its three biochemical activities.Citation3 First, it senses misfolded proteins in the ER and undergoes oligomerization. Second, the oligomerized Ire1p undergoes autophosphorylation through its kinase domain which activates its third activity as an endoribonuclease. The endoribonuclease activity of Ire1p splices the intron from the mRNA of Hac1p, it only known substrate, allowing its efficient translation (). Hac1p, a b-Zip family transcription factor, then translocates to the nucleus and activates a large transcriptional program to compensate for the accumulation of misfolded proteins or other causes of ER stress.Citation4
Initially, the role of UPR was thought to be largely limited to increasing the cells ability to process misfolded proteins, but it is now clear that UPR affects a broad number of processes related to secretory pathway homeostasis.Citation5 Examples of such processes include cytokinesis,Citation6 autophagyCitation7 and filamentous growthCitation8 to list but a few. With the broader role of UPR in mind, we hypothesized that UPR and/or ER quality control mechanisms may be required for yeast cell wall integrity. The yeast cell wall is an extracellular organelle and, consequently, is dependent upon a well-functioning secretory pathway to deliver its constituent proteins as well as the enzymes necessary for its biosynthesis. Interest in the biosynthesis of the fungal cell wall is increasing due to its importance as a target for new antifungal drug development. In a recent report,Citation9 we demonstrated that loss of UPR function (Ire1 is not essential in S. cerevisiae) causes cell wall defects and that cell wall stress activates UPR through the cell wall integrity (CWI) MAP kinase signaling pathwayCitation10 (see ). In this addendum, I summarize these findings and present a model for the inter-related cellular responses to ER and cell wall stress in S. cerevisiae.
Null mutation of IRE1 and expression of misfolded proteins show phenotypes similar to mutations in genes involved in cell wall biosynthesis including hypersensitivity to cell wall active drugs, cell wall degrading enzymes, decreased cell wall thickness and increased cellular aggregation. Since these phenotypes suggest a functional role for UPR and ER quality control in maintaining cell wall integrity, we next asked if cell wall stress activates UPR. Indeed, cells treated with cell wall active molecules or containing mutations that affect cell wall integrity show increased UPR activity based on β-galactosidase reporters of UPR activation and the appearance of spliced Hac1p by RT-PCR. This cell wall stress-induced UPR activation is dependent on Ire1p, indicating that it occurs via the traditional pathway and does not involve an alternative mechanism.
Cell wall stress-induced transcriptional responses are primarily regulated by the CWI MAP kinase pathway (see ). Therefore, we tested the dependence of cell wall stress-induced UPR on components of the CWI pathway. Calcofluor white induces UPR in a process that is dependent on Mid2p, a putative cell wall stress sensor; Mpk1/Slt2p, the MAPK of the CWI pathway; and Swi6p, a transcriptional regulator involved in cell cycle and cell wall-related processes.Citation11 Epistasis analysis revealed that the CWI pathway, Swi6p and Ire1p function in a linear pathway that transmits cell wall stress signals to the UPR.
Although Swi6p functions as a transcriptional regulator, it apparently does not have intrinsic DNA-binding activity and is dependent on other DNA-binding proteins to modulate transcription. Interestingly, Swi6p-mediated UPR activation is independent of any known Swi6p binding partners (Swi4p, Stb1p or Mbp1). At this point, it is unclear if Swi6p-mediates a transcriptional response that indirectly activates UPR or if a more direct mechanism connects Swi6p to Ire1p. Regardless of which alternative is operative, this function of Swi6p is likely to involve a novel mechanism and is currently the subject of active investigation in our laboratory.
Prior to our demonstration that cell wall stress activates UPR, two groups had showed that ER stress activates the CWI pathway.Citation12,Citation13 In contrast to the linear pathway connecting a cell wall stress signal to UPR, ER stress activates UPR and CWI signaling through two parallel circuits (). This difference is most clearly demonstrated by the facts that Cheng et al. showed that mpk1Δ ire1Δ double mutants display synergistic hypersensitivity to ER stress relative to each single mutant,Citation13 while we found that the double mutant was as sensitive to cell wall stress as mpk1Δ and, thus, displayed epistasis.Citation9 Recently, Cohen et al. have shown that the Hos2/Set3 deacetylase complex mediates transmission of the ER stress signal to Slt2/Mpk1.Citation14
Teleologically, the inter-relationship between yeast ER and cell wall stress responses seems to serve a logical purpose. During cell wall stress, the delivery of misfolded or defective proteins to the cell wall would be particularly damaging and, consequently, the cell increases its capacity to process misfolded proteins to decrease the likelihood that such proteins are incorporated into the cell wall. Furthermore, the cell wall stress response leads to the upregulation of a wide variety of cell wall proteins and may also increase the total protein flux through the ER, leading to additional ER stress. Conversely, during ER stress, the delivery of misprocessed proteins to the cell wall may be more likely and, consequently, the cell wall stress response is activated to buffer the cell against defects in cell wall biosynthesis. Together, the coordination of cell wall and ER stress responses protect the cell from the damaging effects of two stressors that ultimately could lead to cell death by causing a defective cell wall.
The connection of cell wall stress and ER stress is not limited to S. cerevisiae and has been shown in two of the most important human fungal pathogens, Candida albicans and Aspergillus fumigatus. Mutations of HAC1 homologues in both of these organisms lead to increased susceptibility to the clinically used, cell wall-directed antifungal caspofungin,Citation15,Citation16 suggesting that this connection could be exploited to develop more effective antifungal drugs or combinations of drugs. Therefore, further study of the mechanisms that regulate the coordination of ER and cell wall stress responses in fungi should not only shed light on basic principles of signaling pathway regulation but could also lead to new approaches to the treatment of fungal infections.
Figures and Tables
Figure 1 Inter-relationship between ER and cell wall stress response in yeast. Cell wall and ER stress both activate cell wall and unfolded protein (UPR) genes through the cell wall integrity MAP kinase pathway and UPR pathways. Pathways with dashed lines involve intermediate steps with unclear mechanisms.
Addendum to:
References
- Spear ED, Ng DTW. The unfolded protein response: no longer just a special teams player. Traffic 2001; 2:515 - 523
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 2007; 8:519 - 529
- Cox JS, Walter P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell 1996; 87:391 - 404
- Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analysis reveals an essential coordination between the unfolded protein response and ER-associated degradation. Cell 2000; 101:249 - 258
- Cox JS, Chapman RE, Walter P. The unfolded protein response coordinates the production of endoplasmic reticulum protein and endoplasmic reticulum membrane. Mol Biol Cell 1997; 8:1805 - 1814
- Bicknell AA, Babour A, Federovitch CM, Niwa M. A novel function in cytokinesis reveals a housekeeping function for the unfolded protein response. J Cell Biol 2007; 177:1017 - 1027
- Bernales S, McDonald KL, Walter P. Autophagy couterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol 2006; 4:423
- Schoder M, Chang JS, Kaufman RJ. The unfolded protein response represses nitrogen-starvation induced developmental differentiation in yeast. Genes Dev 2000; 14:2962 - 2975
- Scrimale T, DiDone L, de Mesy Bentley KL, Krysan DJ. The unfolded protein response is induced by the cell wall integrity mitogen-activated protein kinase pathway and required for cell wall integrity in Saccharomyces cerevisiae. Mol Biol Cell 2009; 20:164 - 175
- Levin DE. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 2005; 69:262 - 291
- Sedgwick SG, Taylor IA, Adam AC, Spanos A, Howell S, Morgan BA, et al. Structural and functional architecture of the yeast cell cycle-transcription factor Swi6. J Mol Biol 1998; 281:763 - 775
- Bonilla M, Cunningham KW. Mitogen-activated protein kinase stimulation of Ca2+ signaling is required for survival of endoplasmic reticulum stress in yeast. Mol Biol Cell 2003; 14:4296 - 4305
- Chen Y, Feldman DE, Deng C, Brown JA, De Giacomo AF, Gaw AF, et al. Identification of mitogen-activated protein kinase signaling pathways that confer resistance to endoplasmic reticulum stress in Saccharomyces cerevisiae. Mol Cancer Res 2005; 3:669 - 677
- Cohen TJ, Mallory MJ, Strich R, Yao TP. Hos2p/Set3p deacetylase complex signals secretory stress through the Mpk1p cell integrity pathway. Eukaryot Cell 2008; 7:1191 - 1199
- Wimalasena TT, Enjalbert B, Guillemette T, Plumridge A, Budge S, Yin ZT, et al. Impact of unfolded protein response upon genome-wide expression patterns, and the role of Hac1 in polarized growth, of Candida albicans. Fungal Genet Biol 2008; 45:1235 - 1247
- Sonmez K, Zaveri NT, Kerman IA, Burke S, Neal CR, Xie X, et al. 2009 Evolutionary sequence modeling for discovery of peptide hormones. PLoS Comput Biol 5:1 e1000258; http://dx.doi.org/10.1371/journal.pcbi.1000258