Abstract
Plant reoviruses in insect vector cells are sequestered in spherical multivesicular compartments. We demonstrated previously that the plant-infecting reovirus Rice dwarf virus (RDV) exploits multivesicular compartments for the transport and release of viral particles from infected insect vector cells. These multivesicular compartments contain small vesicles and, morphologically, they resemble previously reported endosomal multivesicular bodies (MVBs) exploited by enveloped RNA viruses during budding from the plasma membrane of infected cells. Electron microscopy revealed that, at a late stage of infection, RDV virions are released, together with small vesicles similar to secreted vesicles (exosomes), from infected cells. The incorporation of lysosomes into the multivesicular compartments raised the possibility that functions of host MVBs are required for the efficient release of RDV virions from infected insect vector cells. An actin-myosin transport system has been shown to mediate the transport of these multivesicular compartments. In this addendum, we provide evidence for the proposed model of release of RDV virions from infected insect vector cells that exploits secretory exosomes derived from MVBs.
Many cytopathological studies of plant reoviruses in infected plants and vector insects were reported in the 1960s and 1970s.Citation1,Citation2 In electron micrographs of thin sections of insect tissues infected with plant reoviruses, viral particles were apparently sequestered at the periphery of viroplasms, in tubular structures and in spherical multivesicular compartments.Citation1,Citation2 In vector cells grown in monolayers (VCMs), Rice dwarf virus (RDV), a phytoreovirus, multiplies and spreads from primarily infected cells to neighboring cells.Citation3,Citation4 RDV is transmitted efficiently to plant hosts after multiplication to high titer in its vector, and infection is inapparent and persistent. The cultured vector cells and the insect vector exhibit similar responses to RDV infection. Both support the multiplication of RDV to a high titer and in both, an asymptomatic, persistent infection develops.Citation3,Citation4
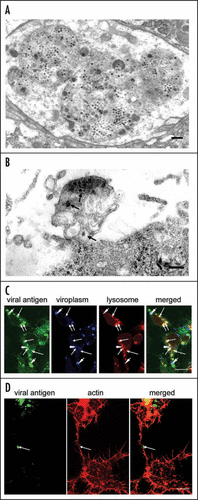
In a recent paper,Citation5 we demonstrated the involvement of multivesicular compartments in infected leafhopper cells in the release of RDV particles from these cells. Our data clearly suggested the existence of a virus-release pathway whereby viral particles, assembled at the periphery of the viroplasm, are engulfed by multivesicular compartments that move to the periphery of cells, where they fuse with the plasma membrane to release viral particles. The multivesicular compartments that contained small vesicles () morphologically resemble endosomal multivesicular bodies (MVBs) that are exploited by retroviruses and certain other enveloped RNA viruses for budding of viral particles from the plasma membrane of infected cells.Citation6–Citation9 MVBs are spherical endosomal organelles and they contain small vesicles that are formed by the inward budding of the limiting membrane into the endosomal lumen.Citation10,Citation11 MVBs fuse with the plasma membrane in an exocytic manner, with release of their contents, including their internal vesicles, into the extracellular space. These released vesicles are known as exosomes. Our electron micrographs indicate that MVB-like multivesicular compartments are involved in the export of RDV particles, which are interspersed among small vesicles that resemble exosomes (), after fusion of the membrane of the compartments with the plasma membrane. The small vesicles resemble exosomes in terms both of location and morphology, and we postulated that multivesicular compartments might fuse with the plasma membrane in an exocytic manner with the resultant release of virions.
To gain a better understanding of virus-host interactions at the late stages of replication of RDV, we examined whether release of RDV into intracellular multivesicular compartments depends on or is independent of MVB functions. We investigated the cellular origin of virus-containing compartments by confocal microscopy, using organelle-specific markers. At a late stage after inoculation with RDV, we immunostained VCMs with RDV-specific antibodies conjugated with fluorescein isothiocyanate (FITC); with antibodies raised against the non-structural protein of RDV known as Pns12, which is a constituent of the viroplasm,Citation12 conjugated with Alexa Fluor 647 carboxylic acid; and the late endosomal or lysosome marker LysoTracker Red DND-99 (Sigma, St. Louis, MO). Then we examined the stained cells by confocal fluorescence microscopy, as described previously.Citation12 Viral particles were assembled in the peripheral region of the viroplasm and formed ring-like structures (), as described previously.Citation12 RDV-specific antibodies also reacted with numerous spherical structures that corresponded to the virus-containing multivesicular compartments ().Citation5 Moreover, immunofluorescence revealed that most of the spherical structures were colocalized with the late-endosomal or lysosomal marker LysoTracker Red (). Since the multivesicular compartments exploited by RDV are similar to MVBs in terms of both origin and morphology, we postulated that MVB functions might also be required for budding of RDV particles into internal compartments with formation of virus-containing small vesicles that resemble exosomes, as is the case for hepatitis B virus, the SARS coronavirus, and certain other viruses.Citation6–Citation9 Nonetheless, conclusive proof that MVB-mediated secretion of exosomes is involved in the release of RDV from infected insect cells requires further analysis.
In our recent paper, we reported that actin filaments and myosin motors affect the morphology and motility of virus-containing MVB-like compartments.Citation5 Such compartments were frequently observed along the surface of actin-based filopodia that connected pairs of insect vector cells (). We proposed, tentatively, that actin polymerization and myosin motors might drive the intracellular trafficking of virus-containing MVB-like compartments and might, eventually, push them through the plasma membrane. The actin cytoskeleton is crucial for the intracellular trafficking of endosomal and lysosomal compartments in cells.Citation13–Citation16 Furthermore, intracellular endosome and lysosomes have been studied extensively in the intercellular transport that occurs along actin-based membrane nanotubules and filopodia.Citation16–Citation20 Therefore, we speculated that virus-containing MVB-like compartments might move along actin-based filopodia that connect pairs of cells, facilitating the cell-to-cell spread of RDV. In plant virus-infected plant cells, Tobacco mosaic virus moves between cells as membrane-bound viral replication complexes (VRCs)Citation21 and an actin-myosin transport system controls the intercellular movement of VRCs.Citation21 This example of the intercellular spread of a plant virus via membrane-bound compartments that are propelled by an actin-myosin transport system fits well with our hypothetical model for the spread of RDV between cells. The intercellular spread among insect vector cells of RDV via actin-based filopodia in the vector insect itself occurs via membrane-bound tubules that are composed of the viral nonstructural protein Pns10 and contain viral particles.Citation22,Citation23 It will be interesting to investigate the ways in which actin-myosin motility systems mediate the movement of two different types of membrane-bound structure in the intercellular spread of RDV. It is possible that the actin cytoskeleton might recognize the Pns10 tubules of RDV as “membranous vesicles” and facilitate their movement to adjacent cells.
The two pathways for the release of RDV particles from infected insect vector cells (in culture and in the vector insect) cause less damage to their host cells than do lytic viruses, such as rotavirus and orthoreovirus to their respective host cells. RDV appears to use components of the vector cell's machinery to reproduce itself but it does so in a manner that does not interfere with the vitality of the organism on which it depends for its subsequent survival. It is tempting to speculate that, during the evolution of this relationship, it became necessary for the virus to regulate the expression of its genome to ensure the survival of its vector, thereby increasing its own chances for survival. The two pathways for the release of mature virions from infected cells provide efficient survival mechanisms that allow RDV to coexist with its insect vector.
Figures and Tables
Figure 1 Virus-containing vesicular compartments at later stages of infection by RDV of cells in monolayer (VCM). (A) Transmission electron micrograph of virus-containing multivesicular compartments that contain small vesicles and that resemble morphologically, multivesicular bodies (MVBs). Bar, 300 nm. (B) Virus-containing exosome-like small vesicles (arrows) were detected in the vicinity of the cell membrane. Bar, 300 nm. (C) Confocal immunofluorescence micrographs of VCM inoculated with RDV showing that most of spherical structures labeled by RDV-specific antibodies, which had been identified as virus-containing multivesicular compartments, were co-localized with the late endosomal or lysosomal marker LysoTracker Red. The ring-like staining of viral antigens and spherical structures composed of Pns12, a component of viroplasms, are indicated by arrowheads. Arrows indicate spherical structures that correspond to lysosomes. Bar, 10 µm. (D) Spherical structures were often observed on the surface of actin-based filopodia that had been labeled by rhodamine phalloidin and visualized by confocal fluorescence microscopy. Bar, 10 µm.
Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research on Priority Areas (Structures of Biological Macromolecular Assemblies), from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by the Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN) to T.O.
Addendum to:
References
- Boccardo G, Milne RG. Morant AF, Harrison BD. Plant reovirus group. CM/AAB Descriptions of Plant Viruses 1984; Farnham Royal, Slough, United Kingdom Commonwealth Agricultural Bureaux 294
- Fukushi I, Shikata E, Kimura I. Some morphological characters of Rice dwarf virus. Virology 1962; 18:192 - 205
- Kimura I. A study of Rice dwarf virus in vector cell monolayers by fluorescent antibody focus counting. J Gen Virol 1986; 67:2119 - 2124
- Kimura I, Omura T. Leafhopper cell cultures as a means for phytoreovirus research. Adv Dis Vector Res 1988; 5:111 - 135
- Wei T, Hibino H, Omura T. Rice dwarf virus is engulfed into and released via vesicular compartments in cultured insect vector cells. J Gen Virol 2008; 89:2915 - 2920
- Watanabe T, Sorensen EM, Naito A, Schott M, Kim S, Ahlquist P. Involvement of host cellular multivesicular body functions in hepatitis B virus budding. Proc Natl Acad Sci USA 2007; 104:10205 - 10210
- Mori Y, Koike M, Moriishi E, Kawabata A, Tang H, Oyaizu H, et al. Human herpesvirus-6 induces MVB formation, and virus egress occurs by an exosomal release pathway. Traffic 2008; 9:1728 - 1742
- Nguyen DG, Booth A, Gould SJ, Hildreth JE. Evidence that HIV budding in primary macrophages occurs through the exosome release pathway. J Biol Chem 2003; 278:52347 - 52354
- Calistri A, Salata C, Parolin C, Palù G. Role of multivesicular bodies and their components in the egress of enveloped RNA viruses. Rev Med Viro 2009; 19:31 - 45
- Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nature Rev Mol Cell Biol 2004; 5:317 - 323
- Denzer K, Kleijmeer MJ, Heijnen HFG, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci 2000; 113:3365 - 3374
- Wei T, Shimizu T, Hagiwara K, Kikuchi A, Moriyasu Y, Suzuki N, et al. Pns12 protein of Rice dwarf virus is essential for formation of viroplasms and nucleation of viral-assembly complexes. J Gen Virol 2006; 87:429 - 438
- Voigt B, Timmers AC, Samaj J, Hlavacka A, Ueda T, Preuss M, et al. Actin-based motility of endosomes is linked to the polar tip growth of root hairs. Eur J Cell Biol 2005; 84:609 - 621
- Sultana H, Rivero F, Blau-Wasser R, Schwager S, Balbo A, Bozzaro S, et al. Cyclase-associated protein is essential for the functioning of the endo-lysosomal system and provides a link to the actin cytoskeleton. Traffic 2005; 6:930 - 946
- Styers ML, Salazar G, Love R, Peden AA, Kowalczyk AP, Faundez V. The endo-lysosomal sorting machinery interacts with the intermediate filament cytoskeleton. Mol Biol Cell 2004; 15:5369 - 5382
- Reed BC, Cefalu C, Bellaire BH, Cardelli JA, Louis T, Salamon J, et al. GLUT1CBP (TIP2/GIPC1) interactions with GLUT1 and myosin VI: evidence supporting an adapter function for GLUT1CBP. Mol Biol Cell 2005; 16:4183 - 4201
- Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science 2004; 303:1007 - 1010
- Scott G, Leopardi S, Printup S, Madden BC. Filopodia are conduits for melanosome transfer to keratinocytes. J Cell Sci 2002; 115:1441 - 1451
- Vidulescu C, Clejan S, O'connor KC. Vesicle traffic through intercellular bridges in DU 145 human prostate cancer cells. J Cell Mol Med 2004; 8:388 - 396
- Onfelt B, Nedvetzki S, Benninger RK, Purbhoo MA, Sowinski S, Hume AN, et al. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J Immunol 2006; 177:8476 - 8483
- Kawakami S, Watanabe Y, Beachy RN. Tobacco mosaic virus infection spreads cell-to-cell as intact replication complexes. Proc Natl Acad Sci USA 2004; 101:6291 - 6296
- Wei T, Kikuchi A, Moriyasu Y, Suzuki N, Shimizu T, Hagiwara K, et al. The spread of Rice dwarf virus among cells of its insect vector exploits virus-induced tubular structures. J Virol 2006; 80:8593 - 8602
- Wei T, Shimizu T, Omura T. Endomembranes and myosin mediate assembly into tubules of Pns10 of Rice dwarf virus and intercellular spreading of the virus in cultured insect vector cells. Virology 2008; 372:349 - 356