Abstract
Parasitoid wasps in search for plant-feeding hosts display typical patterns of behavior. The braconid Cotesia marginiventris, which parasitizes young caterpillars, is guided by herbivore-induced plant volatiles to an infested plant. On the plant, the female wasp searches for further chemical residues (kairomones) originating directly from the host. We showed that caterpillars leave minute amounts of treacherous chemical footprints while walking over a plant surface. Female wasps are able to detect these residues for up to two days after their hosts have left the site. Analyses of the caterpillar footprints revealed that these consisted of linear and monomethyl-branched alkanes as well as few minor unidentified compounds. A reconstructed blend of the major footprint compounds, consisting of linear C21-C32 alkanes, induced characteristic antennation behavior. However, the artificial blend was less attractive than the original one suggesting a role for additional minor compounds in recognizing former caterpillar presence. Previous investigations using wax mutants of barley showed that the physico-chemical traits of the epicuticular leaf wax can modulate the parasitoids’ response to host footprints. We hypothesize that long-chain hydrocarbons of insect and plant cuticular origin are important mediators of insect-plant interactions and believe that their role in modulating trophic cascades still awaits full appreciation.
Female parasitoids are faced with the challenging task of finding a suitable host that can be exploited as a food source by their offspring. The process of host foraging usually consists of several phases known as host habitat location, host location, host recognition and host acceptance.Citation1,Citation2 In each phase volatile or non-volatile chemical cues play an important role (). During the last two decades, volatiles that are actively released by the herbivore-infested plant have received considerable attention. These compounds are able to summon parasitoid wasps and are thus considered as a plant's cry for help. In the second phase of the host location sequence, parasitoids search for reliable chemical signals that originate directly from a potential host. Known sources of host location cues are e.g., host silk, feces, pheromones or defense secretions.Citation3 So far, only limited evidence exists showing that parasitoids, in analogy to predatory mammals, can track down their victims by eavesdropping on chemical footprints left on the substrate.Citation4–Citation6 In our recent publication we investigated the braconid wasp Cotesia marginiventris and its host Spodoptera frugiperda, a popular model parasitoid-host system in many plant volatile studies.Citation7 Combined behavioral experiments and chemical analyses were used to reveal the presence, persistence and chemistry of footprints left by caterpillars while walking over barley leaves.
To test whether caterpillars leave chemical footprints while moving over a plant surface and to assess for how long parasitoids are able to detect these compounds, a clip cage was attached to the second leave of a barley plant (Hordeum vulgare cv. Bonus). The cage contained four-second instar larvae of Spodoptera frugiperda, which were allowed to walk on the leaf for 15 min. After removing the caterpillars, all leaves were scrutinized. Those with bite marks, feces or silk were discarded. Depending on the experiment, the leaves were cut off after a period of 0 h, 24 h, 48 h or 72 h and placed individually in Petri dishes together with an untreated control leaf. Characteristic antennation behavior (as an indicator of chemosensation) of single C. marginiventris wasps was observed for 10 min. Our choice tests showed that leaf segments from which the caterpillars had just been removed, elicited strong antennation behavior in the female wasps. The intensity of this behavior abated the more time between the contact of host and plant had passed and demonstrated that the parasitoids were able to detect the caterpillar footprints for up to two days.
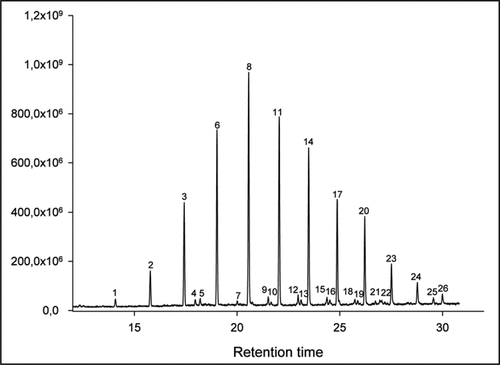
In order to identify the inducing compounds, 100 caterpillars were allowed to walk over clean glass slides covered with small Petri dishes. The glass was rinsed with hexane and the extract was concentrated under a flow of nitrogen. Hexane washings from glass slides without caterpillars were used as controls. We then analyzed the footprint compounds by gas chromatography combined with mass spectrometry. The extracts of the footprints were also compared with washings from the caterpillar cuticle to gain information on the footprints' origin. We were able to identify 21 out of 26 compounds in the footprint extract (). The majority (90% of total) of the compounds were linear alkanes with chain lengths ranging from 21 to 32 carbon atoms with pentacosane (13.5%), hexacosane (10.4%) and heptacosane (10.3%) being the most abundant ones. In addition, minor amounts of monomethyl-branched compounds (4C24-4MeC28 and 3MeC25-3MeC28) were present, as well. Since extracts of footprint and cuticle were almost identical, we presumed that the identified compounds were of cuticular origin and we hypothesized that prolegs and claspers of the caterpillar were the sources of the chemical signal. These extremities and the three pairs of true legs were the only parts in contact with the substrate.
Subsequently, behavioral assays were carried out to confirm the antennation-inducing role of the major footprint compounds. We observed the parasitoids' responses to the original hexane extract of caterpillar footprints and to a partially reconstructed blend of the footprint extract. The artificial blend consisted of all main compounds, i.e., the linear alkanes, which were commercially available. The ratios and quantities of the compounds were the same as in the original footprint extract. Females of C. marginiventris antennated significantly longer on leaves treated with one of the extracts compared to hexane-treated control leaves. However, the parasitoids responded decidedly stronger to the original footprint extract compared to the reconstructed blend suggesting that minor compounds were also important for host recognition. Our observation was consistent with studies on Nezara viridula and its egg parasitoid Trissolcus basalis where a reconstructed blend of straight-chain hydrocarbons extracted from the host also induced only weak arrestment responses in T. basalis females.Citation4 Methyl-branched alkanes and alkenes often occur in insect hydrocarbons and sometimes have behavioral activity as contact pheromones.Citation8 In plant waxes, however, they can be detected merely in trace amounts if at all. This and the fact that n-alkanes are common plant wax compounds suggest that minor constituents may enable the wasps to detect insect hydrocarbons against a background of similar plant hydrocarbons. However, in the experiments described here, barley leaves were used as a surface for caterpillars to walk on. The epicuticular wax of this plant contains very small proportions of n-alkanes with heptacosane (nC27) as the only overlapping compound that was also present in the chemical footprint extract.Citation9
Anyway, the role of plant wax composition in attenuating or enhancing footprint detection has hardly been assessed and so far only two studies using wax mutants have demonstrated that insect-derived chemical signals can be better perceived on some wax surfaces than on others.Citation9,Citation10 We expect that future research will establish the full significance of residual chemicals and correspondingly the role of the plant surface in regulating insect behavior.
Figures and Tables
Figure 1 Host location behavior of the parasitoid Cotesia marginiventris. (1) Female wasp is attracted to an infested plant by herbivore-induced plant volatiles (green leaf volatiles, terpenes, shikimic acid-derived compounds). (2) Parasitoid searches plant for non-volatile chemical cues such as caterpillar footprints (or feces, silk), indicating the presence of a host. (3) Host recognition and oviposition into the caterpillar.
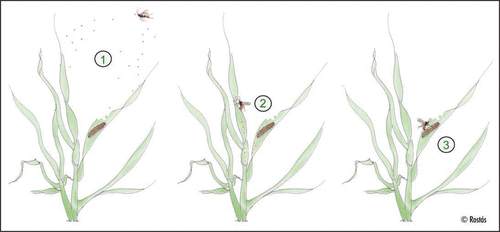
Figure 2 Chromatogram of footprint extract from caterpillars of Spodoptera frugiperda. (1) n-heneicosane (nC21), (2) n-docosane (nC22), (3) n-tricosane (nC23), (4) unknown, (5) unknown, (6) n-tetracosane (nC24), (7) 4-methyl tetracosane (4MeC24), (8) n-pentacosane (nC25), (9) 4-methyl pentacosane (4MeC25), (10) 3-methyl pentacosane (3MeC25), (11) n-hexacosane (nC26), (12) 4-methyl hexacosane (4MeC26), (13) 3-methyl hexacosane (3MeC26), (14) n-heptacosane (nC27), (15) 4-methyl heptacosane (4MeC27), (16) 3-methyl heptacosane (3MeC27), (17) n-octacosane (nC28), (18) 4-methyl octacosane (4MeC28), (19) 3-methyl octacosane (3MeC28), (20) n-nonacosane (nC29), (21) unknown, (22) unknown, (23) n-triacontane (nC30), (24) n-hentriacontane (nC31), (25) unknown, (26) n-dotriacontane (nC32).
Acknowledgements
Laura Rostas provided the drawing. Financial support was obtained from the Deutsche Forschungsgemeinschaft (SFB 567, TP B9).
Addendum to:
References
- Steidle JLM, van Loon JJA. Dietary specialization and infochemical use in carnivorous arthropods: testing a concept. Ent Exp Appl 2003; 108:133 - 148
- Vinson SB. The general host selection behavior of parasitoid hymenoptera and a comparison of initial strategies utilized by larvaphagous and oophagous species. Biol Control 1998; 11:79 - 96
- Godfray HCJ. Parasitoids: Behavioral and Evolutionary Ecology 1994; Princeton, NJ Princeton University Press
- Colazza S, Aquila G, De Pasquale C, Peri E, Millar JG. The egg parasitoid Trissolcus basalis uses n-nonadecane, a cuticular hydrocarbon from its stink bug host Nezara viridula, to discriminate between female and male hosts. J Chem Ecol 2007; 33:1405 - 1420
- Conti E, Salerno G, Bin F, Williams HJ, Vinson SB. Chemical cues from Murgantia histrionica eliciting host location and recognition in the egg parasitoid Trissolcus brochymenae. J Chem Ecol 2003; 29:115 - 130
- Klomp H. Parasitic wasps as sleuth-hounds—Response of an ichneumon wasp to the trail of its host. Neth J Zool 1981; 31:762 - 772
- Rostás M, Wölfling M. Caterpillar footprints as host location kairomones for Cotesia marginiventris: persistence and chemical nature. J Chem Ecol 2009; 35:20 - 27
- Ginzel MD, Millar JG, Hanks LM. (Z)-9-Pentacosene—contact pheromone of the locust borer, Megacyllene robiniae. Chemoecology 2003; 13:135 - 141
- Rostás M, Ruf D, Zabka V, Hildebrandt U. Plant surface wax affects parasitoid's response to host footprints. Naturwissenschaften 2008; 95:997 - 1002
- Rutledge CE, Eigenbrode SD, Ding H. A plant surface mutation mediates predator interference among ladybird larvae. Ecol Entomol 2008; 33:464 - 472