Abstract
The extracellular matrix protein Reelin, secreted by Cajal-Retzius (CR) cells in the marginal zone (MZ) of the cerebral cortex, is important for neuronal migration during development. Two lipoprotein receptors for Reelin have been identified, apolipoprotein E receptor 2 (ApoER2) and the very low density lipoprotein receptor (VLDLR). The binding of Reelin to these receptors induces tyrosine phosphorylation of an adapter protein, disabled 1 (Dab1) by src family kinases (SFKs). In the Reelin-deficient mutant reeler, cortical lamination is inverted with many neurons invading the marginal zone and others that are unable to migrate to their destinations and accumulate underneath their predecessors, suggesting a role for Reelin signaling in dynamic cytoskeletal reorganization. At present these effects of Reelin are poorly understood. In our recent study, we showed that Reelin induces serine3 phosphorylation of n-cofilin, an actin-depolymerizing protein promoting the disassembly of F-actin. Phosphorylation of cofilin renders it unable to depolymerize F-actin, thus stabilizing the cytoskeleton. We provided evidence for ApoER2, Dab1, SFKs, and phosphatidylinositol-3-kinase (PI3K) to be involved in Reelin-induced cofilin phosphorylation. We found that phosphorylation of cofilin occurs in the leading processes of radially migrating neurons as they grow towards the Reelin-containing marginal zone. By cofilin phophorylation, Reelin may act as a stop signal for radially migrating neurons.
During brain development, cortical neurons are born near the ventricle and migrate radially towards the Reelin-containing marginal zone. The six-layered cortex is established according to an inside-out patterning with early generated neurons occupying the deep layers and late-born neurons bypassing their predecessors to reach more superficial layers.Citation1 In contrast, in reeler mutant mice lacking Reelin, cortical lamination is inverted.Citation2 Neurons are unable to bypass their predecessors and pile up underneath. In wild-type animals the marginal zone is cell-sparse, whereas it is densely populated in reeler mutants. Thus, Reelin has been suggested to act as a stop signalCitation3–Citation6 or positional signalCitation4,Citation7 for radially migrating neurons. However, the mechanism of Reelin's action on the migration process is still poorly understood.
Many studies have shown that Reelin binds to ApoER2 and VLDLR in the membranes of the migrating neurons.Citation8,Citation9 Binding of Reelin to ApoER2 and VLDLR induces tyrosine phosphorylation of an adapter protein, Dab1, which interacts with the intracellular domains of these receptors.Citation9–Citation11 It has been shown that non-receptor tyrosine kinases, SFKs, phosphorylate Dab1 upon Reelin binding.Citation12,Citation13 Another kinase in the Reelin signaling pathway is PI3K. Blocking of PI3K by LY294002 was found to induce inverted cortical layering reminiscent of the reeler phenotype in embryonic slice cultures.Citation14
Mutations in the genes encoding for Reelin, its receptors, Dab1 or SFKs such as fyn and src, cause a common reeler-like phenotype.Citation9,Citation15,Citation16 In cultures of the dentate gyrus, the reeler phenotype could be rescued by wild-type co-culture, and the rescue was found to be mediated by lipoprotein receptors for Reelin and Dab1.Citation17 Together these studies indicate that Reelin, its lipoprotein receptors, Dab1 and PI3K are all members of the same signaling pathway.
How to explain the function of Reelin in the control of neuronal migration? How does Reelin control the actin cytoskeleton? To address these questions, it may be useful to have a look at the modes of neuronal migration during development. Two different modes of radial migration have been described: somal translocation and glia-guided migration.Citation18,Citation19 Somal translocation predominates during the early phases of cortical development with the leading process attaching the marginal zone; thereafter, when the migratory route to the cortical plate has increased, postmitotic neurons born in the ventricular zone migrate towards the pial surface with the guidance of radial glial fibers extending from the subventricular zone to the pial surface. As soon as the leading processes of late generated neurons approach the Reelin-containing marginal zone, the neurons switch from locomotion to somal translocation to terminate the migrational process.Citation6,Citation18
Essentially, nuclear translocation consists of three steps: extension of a lamellipodium, forward movement of the cell body, and retraction of the trailing process. These dynamic cytoskeletal changes involve the actin cytoskeleton of migrating cells. The actin cytoskeleton (F-actin) at the leading edge is subjected to an organized process of assembly/disassembly to allow for the changes in cell shape required for the cell to move forward.Citation20 These cytoskeletal changes are regulated by actin-binding proteins such as cofilin, an essential regulator of actin dynamics.Citation21 Cofilin acts as an actin-depolymerizing protein that has severing activity; it binds to F-actin and promotes its disassembly. Cofilin is located at the membrane of the leading edge of migrating cells, promotes lamellipodia formation by providing actin monomers,Citation22 and is required for directional migration.Citation23,Citation24 In response to extracellular stimuli, cofilin is phosphorylated at serine 3 by LIMK1.Citation25 Phosphorylation makes cofilin unable to disassemble F-actin. As a result, actin dynamics and subsequent process extension are inhibitedCitation26,Citation27 and the cytoskeleton is stabilized. Such a scenario has to be assumed for a variety of cells including migrating neurons of the cerebral cortex.Citation28
In our recent studies, we asked whether Reelin acts on cofilin phosphorylation. We performed a western blot analysis for p-cofilin and found that phosphorylated n-cofilin is severely reduced in E18 reeler cortices when compared to wild-type or heterozygous mice. Exogenous Reelin increased phosphorylated cofilin in reeler mutant cortices, and this increase in p-cofilin was accompanied by an increase of p-LIMK1, the cofilin kinase. In addition, in the presence of SFK inhibitor PP2, Reelin-induced p-cofilin was downregulated. In the presence of inhibitors of PI3K, LY294002 and Wortmannin, Reelin-induced p-cofilin was abolished. We also found that in Dab1 and ApoER2 mutant mice Reelin application could not induce the phosphorylation of cofilin. Together these results suggested that Reelin induces phosphorylation of cofilin in the cortex, and that this process involves ApoER2, Dab1, SFKs and PI3K.
Our next question was: Where would we find phospho-cofilin? To address this question, we immunostained cortical slices from E17.5 wild-type or reeler mice and found that p-cofilin is located in the marginal zone, i.e., the zone of Reelin-synthesizing CR cells. High-power magnification revealed that p-cofilin is present in the leading processes of radially migrating neurons as they approach the Reelin-containing marginal zone ().
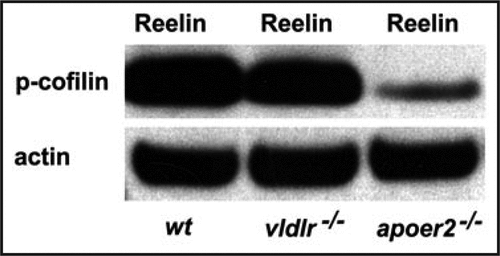
While p-cofilin was found strongly reduced in ApoER2 knockout mice, it appeared almost normal in VLDLR mutants, comparable with the level of phosphorylation of Dab1 in these mutants ().Citation29 We concluded that ApoER2 and VLDLR play divergent roles in the migration of cortical neurons.Citation30 In ApoER2 mutants late generated neurons are unable to bypass early-generated cells and remain close to the subventricular zone. In contrast, in VLDLR mutants, many neurons invade the marginal zone. We hypothesize that Reelin binds to ApoER2 receptors on the surface of migrating neurons and in turn induces phosphorylation of cofilin to stabilize the leading process. Reelin binding to VLDLR receptors may be involved in the arrest of the nucleus in the terminal phase of migration by nuclear translocation.
Abbreviations
| CR | = | Cajal-Retzius |
| MZ | = | marginal zone |
| ApoER2 | = | apolipoprotein E receptor 2 |
| VLDLR | = | very low density lipoprotein receptor |
| Dab1 | = | disabled 1 |
| SFKs | = | Src family kinases |
| p-cofilin | = | phospho-cofilin |
| p-LIMK1 | = | phospho-Lim kinase 1 |
| n-cofilin | = | non-muscle cofilin |
| F-actin | = | filamentous actin |
| PI3K | = | phosphatidylinositol-3-kinase |
Figures and Tables
Figure 1 Leading processes of radially migrating neurons are anchored to the marginal zone of the cortex. (A) DiI labeling shows that late generated neurons extend their leading processes towards the pial surface. (B) Double immunostaining for p-cofilin (green) and Reelin (red) labels the leading processes of late generated neurons in superficial cortical layers and Reelin-synthesizing Cajal-Retzius cells in the marginal zone. Reelin-synthesizing Cajal-Retzius cells are not labeled for p-cofilin. Scale bars, 40 µm.
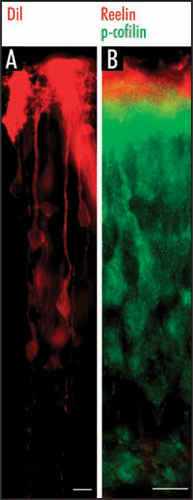
Figure 2 Reelin-induced phosphorylation of n-cofilin involves ApoER2 and VLDLR. In cortical lysates prepared from E18 wild-type mice and treated with recombinant Reelin the protein levels of phosphorylated cofilin (p-cofilin) are much higher when compared to those from ApoER2-/- mice. Levels of p-cofilin were only slightly reduced in VLDLR-/- tissue. Actin was used as a loading control.
References
- Angevine JB, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature 1961; 192:766 - 768
- Caviness VS Jr. Patterns of cell and fiber distribution in the neocortex of the reeler mutant mouse. J Comp Neurol 1976; 170:435 - 447
- Rakic P, Caviness VS Jr. Cortical development: view from neurological mutants two decades later. Neuron 1995; 14:1101 - 1104
- Frotscher M. Cajal-Retzius cells, Reelin and the formation of layers. Curr Opin Neurobiol 1998; 8:570 - 575
- Förster E, Jossin Y, Zhao S, Chai X, Frotscher M, Goffinet AM. Recent progress in understanding the role of Reelin in radial neuronal migration, with specific emphasis on the dentate gyrus. Eur J Neurosci 2006; 23:901 - 909
- Cooper JA. A mechanism for inside-out lamination in the neocortex. Trends Neurosci 2008; 31:113 - 119
- Zhao S, Chai X, Förster E, Frotscher M. Reelin is a positional signal for the lamination of dentate granule cells. Development 2004; 131:5117 - 5125
- D'Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T. Reelin is a ligand for lipoprotein receptors. Neuron 1999; 24:471 - 479
- Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, et al. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell 1999; 97:689 - 701
- Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, et al. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron 1999; 24:481 - 489
- Howell BW, Herrick TM, Cooper JA. Reelin-induced tyrosine phosphorylation of disabled 1 during neuronal positioning. Genes Dev 1999; 13:643 - 648
- Arnaud L, Ballif BA, Förster E, Cooper JA. Fyn tyrosine kinase is a critical regulator of Disabled-1 during brain development. Curr Biol 2003; 13:9 - 17
- Bock HH, Herz J. Reelin activates SRC family tyrosine kinases in neurons. Curr Biol 2003; 13:18 - 26
- Bock HH, Jossin Y, Liu P, Förster E, May P, Goffinet AM, et al. Phosphatidylinositol 3-kinase interacts with the adapter protein Dab1 in response to Reelin signaling and is required for normal cortical lamination. J Biol Chem 2003; 278:38772 - 38779
- Sheldon M, Rice DS, D'Arcangelo G, Yoneshima H, Nakajima K, Mikoshiba K, et al. Scrambler and yotari disrupt the disabled gene and produce a reeler-like phenotype in mice. Nature 1997; 389:730 - 733
- Kuo G, Arnaud L, Kronstad-O'Brien P, Cooper JA. Absence of Fyn and Src causes a reeler-like phenotype. J Neurosci 2005; 25:8578 - 8586
- Zhao S, Chai X, Bock HH, Brunne B, Förster E, Frotscher M. Rescue of reeler phenotype in the dentate gyrus by wild-type coculture is mediated by lipoprotein receptors for Reelin and Dab1. J Comp Neurol 2006; 495:1 - 9
- Nadarajah B, Brunstrom JE, Grutzendler J, Wong RO, Pearlman AL. Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci 2001; 4:143 - 150
- Hatanaka Y, Hisanaga S, Heizmann CW, Murakami F. Distinct migratory behavior of early- and late-born neurons derived from the cortical ventricular zone. J Comp Neurol 2004; 479:1 - 14
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003; 112:453 - 465
- Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Biol 1999; 15:185 - 230
- Kiuchi T, Ohashi K, Kurita S, Mizuno K. Cofilin promotes stimulus induced lamellipodium formation by generating an abundant supply of actin monomers. J Cell Biol 2007; 177:465 - 476
- Dawe HR, Minamide LS, Bamburg JR, Cramer LP. ADF/cofilin controls cell polarity during fibroblast migration. Curr Biol 2003; 13:252 - 257
- Ghosh M, Song X, Mouneimne G, Sidani M, Lawrence DS, Condeelis JS. Cofilin promotes actin polymerization and defines the direction of cell motility. Science 2004; 304:743 - 746
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, et al. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 1998; 393:805 - 809
- Moriyama K, Iida K, Yahara I. Phosphorylation of Ser-3 of cofilin regulates its essential function on actin. Genes Cells 1996; 1:73 - 86
- Zebda N, Bernard O, Bailly M, Welti S, Lawrence D. Phosphorylation of ADF/cofilin abolishes EGF-induced actin nucleation at the leading edge and subsequent lamellipod extension. J Cell Biol 2000; 151:1119 - 1127
- Bellenchi GC, Gurniak CB, Perlas E, Middei S, Ammassari-Teule M, Witke W. N-cofilin is associated with neuronal migration disorders and cell cycle control in the cerebral cortex. Genes Dev 2007; 21:2347 - 2357
- Bock HH, Jossin Y, May P, Bergner O, Herz J. Apolipoprotein E receptors are required for Reelin-induced proteasomal degradation of the neuronal adaptor protein disabled-1. J Biol Chem 2004; 279:33471 - 33479
- Hack I, Hellwig S, Junghans D, Brunne B, Bock HH, Zhao S, et al. Divergent roles of ApoER2 and VLDLR in the migration of cortical neurons. Development 2007; 134:3883 - 3891