Abstract
Nucleic acid cellular uptake into endosomes is critical in eliciting nucleotide-sensing toll-like receptors (TLRs) innate immune responses. ADP-ribosylation factor 6 (ARF6) is a member of the Ras superfamily, which is critical to a wide variety of cellular events including endocytosis. Our previous report indicated that ARF6 plays a critical role in CpG ODN/TLR9-mediated responses. Here, we further explored that the basal level of active ARF6 is nonspecifically responsible for initiation of ODNs uptake, which is relatively CpG motif independent. While the initiation of CpG ODN uptake but not GpC ODN uptake can promote TLR9 responses thereby enhancing ARF6 activation which may lead to further nonspecifically increase of cellular uptake of stimulatory CpG ODN as well as nonstimulatory GpC ODN. Because nucleotide-sensing TLR9 plays a role in contributing to immune diseases, selective activation or inhibition of ARF6 might be useful in certain immunological or therapeutic applications.
Toll like receptors (TLRs) are pattern recognition receptors that interact with components of pathogens and transformed cells to activate the innate immune system and also the adaptive immune system. Bacterial/viral CpG DNA and biological active synthetic oligodeoxynucleotides containing an unmethylated CpG motif (CpG ODN) activate immune responses in a toll-like receptor 9 (TLR9)-dependent manner. Therefore, understanding of the TLR9 signaling pathway will shed light on how the immune response is activated and will benefit the development of specific therapies that can efficiently fight against cancer and infectious diseases.
ADP-ribosylation factors (ARFs) are members of 20 kDa guanine nucleotide-binding proteins within the Ras superfamily of GTPase. There are six related gene products, ARF1 to ARF6, which have been divided into three classes based on sequence homology.Citation1 Class I contains ARF1 to ARF3, class II ARF4 and ARF5, and class III ARF6. The functions of ARF protein are dependent on binding and hydrolyzing GTP, thereby cycling between GTP-bound (ARF-GTP) and GDP-bound (ARF-GDP) forms of the proteins. The active ARF6 localizes on the plasma membrane and has multiple roles in the regulation of cellular functions, including endocytosis and protein trafficking.Citation1 Although the functions of ARF6 have been extensively characterized at the cellular and molecular level, the physiological involvement of ARF6 in CpG ODN/TLR9-mediated signaling remains to be determined. We recently used knockdown and dominant-negative approaches to demonstrate that ARF6 activation plays an important role in TLR9-mediated responses, including NF-κB activation and cytokine production (e.g., IL-6 and TNF-α).Citation2
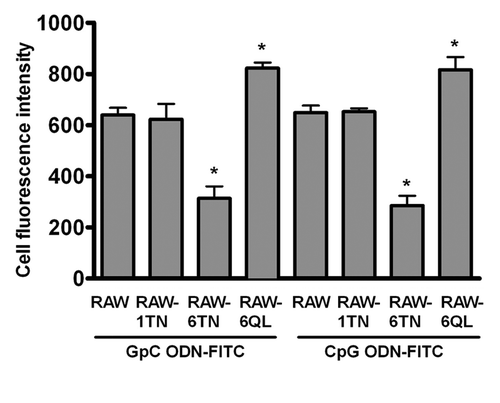
Stimulation of TLR9 by immunostimulatory CpG ODN but not nonstimulatory ODN induces potent Th1-like immune responses that are protective against several infectious agents and immune disorders in animal models.Citation3-Citation5 CpG ODN thus is being evaluated as a potential candidate in immunotherapy of immune-related diseases, and as an adjuvant in vaccine protocols.Citation6-Citation8 Since TLR9 is an intracellular protein, it is apparent that stimulation of immune cells by CpG ODN requires internalization. However, the high efficiency of cellular CpG ODN uptake is thought to be one of challenges that limit its clinical effectiveness, supporting that understanding the mechanism of CpG ODN uptake is crucial. Studies using fluorescently conjugated ODNs indicate that both CpG ODN and nonstimulatory ODNs are internalized nonspecifically, but only stimulatory CpG ODNs activate TLR9 in endosomes.Citation9-Citation11 Furthermore, ODN uptake is dependent on dose, time, and temperature but independent of the CpG motif.Citation12 Accumulated evidence has shown that class III PI3K is specifically involved in TLR9 signaling by regulating the uptake of CpG ODN.Citation9 Our recent findings further suggest that a novel class III PI3K-ARF6 axis pathway mediates TLR9 immune responses by regulating the cellular uptake of CpG ODN.Citation2 However, it is a remaining question whether ARF6 specifically regulates stimulatory CpG ODN uptake, or also affects on nonstimulatory ODN (GpC ODN) uptake. To address this question, we compared the level of CpG ODN-FITC and GpC ODN-FITC uptake in RAW264.7, RAW264.7 cells stably expressing dominant-negative form of ARF6, ARF6T27N, (RAW-ARF6T27N) and RAW-ARF1T31N cells stably expressing dominant-negative form of ARF1, ARF1T31N.Citation13,Citation14 The uptake level of not only CpG ODN-FITC but also GpC ODN-FITC significantly decreased in RAW-ARF6T27N, but not in RAW-ARF1T31N cells as compared with RAW264.7 cells (). In addition, uptake level of the two different ODN-FITCs was increased in RAW-ARF6Q67L; RAW264.7 cells expressing constitutively active human ARF6Citation13; (). These results suggest that ARF6 activation may be nonspecifically involved in cellular uptake of both stimulatory CpG ODN and nonstimulatory GpC ODN.
Figure 1. ARF6 regulates cellular ODN uptake. RAW264.7, RAW-ARF1T31N, RAW-ARF6T27N or RAW-ARF6Q71L cells were incubated with 0.5 μM GpC ODN-FITC or CpG ODN-FITC for 30 min. Cell fluorescence intensity of GpC ODN-FITC or CpG ODN-FITC uptake was measured by flow cytometry. *p < 0.01 for RAW-ARF6T27N or RAW-ARF6Q71Lvs. RAW264.7.
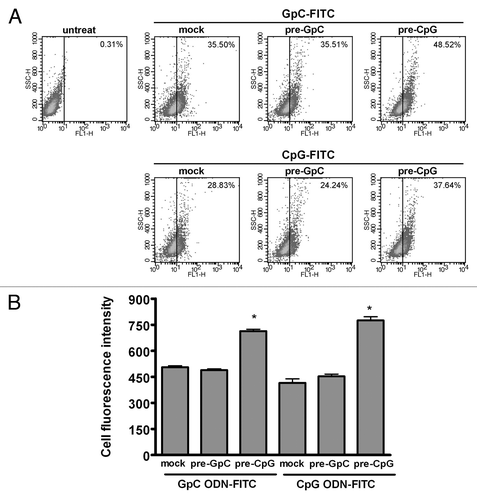
Our previous studies using GST-GGA1 pull down assay have indicated that CpG ODN, but not GpC ODN, activate TLR9 signaling to induce ARF6 activation.Citation2 To further investigate whether CpG ODN induces ARF6 activation, thereby nonspecifically enhancing cellular ODN uptake, the uptake level of CpG ODN-FITC or GpC ODN-FITC was measured in RAW264.7 cells pre-treated with CpG ODN or GpC ODN. Our results showed that CpG ODN pre-treated cells increased uptake level of not only CpG ODN-FITC but also GpC ODN-FITC as compared with endotoxin-free TE buffer treated cells (mock), while the ODN-FITC uptake level was not substantially altered in GpC ODN pre-treated cells (). These results suggest that ARF6 activation induced by CpG ODN may lead to further nonspecifically increase of cellular uptake of either stimulatory CpG ODN or nonstimulatory GpC ODN. Furthermore, the propagating of ARF6 activation advances TLR9 signaling by enhancing stimulatory CpG ODN uptake.
Figure 2. CpG ODN pre-treated cells increase cellular ODN uptake. RAW264.7 cells were pre-treated with either CpG ODN or GpC ODN for 30 min, followed by 0.5 μM GpC ODN-FITC or CpG ODN-FITC for 10 min. (A) The percentages of FITC-positive cells in total cells counted and (B) Cell fluorescence intensity of GpC ODN-FITC or CpG ODN-FITC uptake was measured by flow cytometry. Data represent the mean ± SD of 3 experiments. *p < 0.01 for CpG ODN pre-treated cells vs. GpC ODN pre-treated cells.
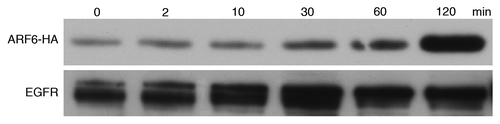
ARF cycles between a GDP-bound inactive state and a GTP-bound active state. GDP-bound ARF is primarily cytosolic, although being weakly associated with membranes, whereas the active GTP-bound form binds tightly to membranes.Citation1 Since endocytosis is involved in CpG ODN uptake and TLR9 signaling, we thus evaluated the level of ARF6 in membrane fraction. Subcellular fractionation that separated total membranes from cytosol were performed initially on a RAW264.7 cells treated with CpG ODN for the indicated times. Western blotting results indicated that a detectable degree of basal level of membrane form was observed in untreated cells; furthermore, the level of ARF6 in membrane fraction was increased after 30 min of CpG ODN stimulation (). These results suggest that active ARF6 translocated from cytosol to plasma membrane, thereby implementing CpG ODN uptake via endocytosis.
Figure 3. CpG ODN induces recruitment of ARF6 to plasma membrane. RAW-ARF6 cells were treated with CpG ODN for the indicated times and then the distribution of ARF6 on plasma membrane was assessed by immunoblot with anti-HA and anti-EGFR (membrane protein marker).
We recently reported that activation of ARF6 increased, while inhibition of ARF6 decreased, CpG ODN uptake and CpG ODN-induced responses, including NF-κB activation and cytokine production. Besides, the amounts of CpG ODN uptake are positively correlated with the cellular activity of ARF6.Citation2 In the present study, our findings suggest that the basal level of active ARF6 is nonspecifically responsible for initiation of ODNs uptake, which is relatively CpG motif independent. Instead, only the initiation of CpG ODN uptake, but not GpC ODN uptake, can promote TLR9 responses thereby enhancing ARF6 activation which is implicated in further increasing ODN uptake. The propagating of ARF6 activation by CpG ODN may thus lead to further increase of CpG ODN uptake which advances TLR9 signaling. Like TLR9, nucleotide-sensing receptors TLR3 and TLR7 are located in intracellular compartments and recognize double-strand RNA and single-strand RNA in endosomes, respectively, to activate innate immune responses.Citation15,Citation16 It thus is intriguing to speculate that ARF6 may modulate TLR3- and TLR7-signaling cascades by regulating their ligand cellular uptake as well. However, the relevance of ARF6-dependent pathway to TLR3- and TLR7-signaling cascades requires further investigation. Because nucleotide-sensing TLRs play roles in contributing to autoimmune diseases such as systemic lupus erythematosus,Citation15,Citation16 no doubt understanding the mechanisms by which ARF6 regulate TLR3, TLR7 and TLR9 is valuable for future autoimmunotherapeutic applications.
| Abbreviations: | ||
| CpG ODN | = | CpG oligodeoxynucleotides |
| TLR9 | = | Toll-like receptor 9 |
| ARF6 | = | ADP-ribosylation factor 6 |
Acknowledgments
This work was supported in part by National Health Research Institutes (Taiwan) intramural grants 99A1-CSPP09-014 (to C.-C.K.) and National Science Council (Taiwan) grant 98-2320-B-400-007-MY3 (to C.-C.K.).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- D’Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol 2006; 7:347 - 58; http://dx.doi.org/10.1038/nrm1910; PMID: 16633337
- Wu JY, Kuo CC. Pivotal role of ADP-ribosylation factor 6 in Toll-like receptor 9-mediated immune signaling. J Biol Chem 2012; 287:4323 - 34; http://dx.doi.org/10.1074/jbc.M111.295113; PMID: 22170068
- Kline JN, Waldschmidt TJ, Businga TR, Lemish JE, Weinstock JV, Thorne PS, et al. Modulation of airway inflammation by CpG oligodeoxynucleotides in a murine model of asthma. J Immunol 1998; 160:2555 - 9; PMID: 9510150
- Shirota H, Sano K, Hirasawa N, Terui T, Ohuchi K, Hattori T, et al. Novel roles of CpG oligodeoxynucleotides as a leader for the sampling and presentation of CpG-tagged antigen by dendritic cells. J Immunol 2001; 167:66 - 74; PMID: 11418633
- Kumagai Y, Takeuchi O, Akira S. TLR9 as a key receptor for the recognition of DNA. Adv Drug Deliv Rev 2008; 60:795 - 804; http://dx.doi.org/10.1016/j.addr.2007.12.004; PMID: 18262306
- Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov 2006; 5:471 - 84; http://dx.doi.org/10.1038/nrd2059; PMID: 16763660
- Krieg AM. Development of TLR9 agonists for cancer therapy. J Clin Invest 2007; 117:1184 - 94; http://dx.doi.org/10.1172/JCI31414; PMID: 17476348
- Hennessy EJ, Parker AE, O’Neill LA. Targeting Toll-like receptors: emerging therapeutics?. Nat Rev Drug Discov 2010; 9:293 - 307; http://dx.doi.org/10.1038/nrd3203; PMID: 20380038
- Häcker H, Mischak H, Miethke T, Liptay S, Schmid R, Sparwasser T, et al. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J 1998; 17:6230 - 40; http://dx.doi.org/10.1093/emboj/17.21.6230; PMID: 9799232
- Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol 2004; 5:190 - 8; http://dx.doi.org/10.1038/ni1028; PMID: 14716310
- Takeshita F, Gursel I, Ishii KJ, Suzuki K, Gursel M, Klinman DM. Signal transduction pathways mediated by the interaction of CpG DNA with Toll-like receptor 9. Semin Immunol 2004; 16:17 - 22; http://dx.doi.org/10.1016/j.smim.2003.10.009; PMID: 14751759
- Guo LH, Schluesener HJ. Binding and uptake of immunostimulatory CpG oligodeoxynucleotides by human neuroblastoma cells. Oligonucleotides 2004; 14:287 - 98; http://dx.doi.org/10.1089/oli.2004.14.287; PMID: 15665596
- Peters PJ, Hsu VW, Ooi CE, Finazzi D, Teal SB, Oorschot V, et al. Overexpression of wild-type and mutant ARF1 and ARF6: distinct perturbations of nonoverlapping membrane compartments. J Cell Biol 1995; 128:1003 - 17; http://dx.doi.org/10.1083/jcb.128.6.1003; PMID: 7896867
- Dascher C, Balch WE. Dominant inhibitory mutants of ARF1 block endoplasmic reticulum to Golgi transport and trigger disassembly of the Golgi apparatus. J Biol Chem 1994; 269:1437 - 48; PMID: 8288610
- Barrat FJ, Coffman RL. Development of TLR inhibitors for the treatment of autoimmune diseases. Immunol Rev 2008; 223:271 - 83; http://dx.doi.org/10.1111/j.1600-065X.2008.00630.x; PMID: 18613842
- Saitoh S, Miyake K. Regulatory molecules required for nucleotide-sensing Toll-like receptors. Immunol Rev 2009; 227:32 - 43; http://dx.doi.org/10.1111/j.1600-065X.2008.00729.x; PMID: 19120473