Abstract
Researchers around the world perform large-scale screens to identify disease-related gene defects in humans. One of the genes of interest is Pericentrin (PCNT), a gene which codes for a large coiled-coil protein with multiple functions in the cell. Recently, we showed that different Pericentrin (Pcnt) splice variants are differentially distributed among sensory tissues of the mouse, emphasizing the importance of a protein‘s spliceome for the function of a cell.
Pericentrin (Pcnt)
Pcnt, a component of the pericentriolar material (PCM), is a highly conserved protein throughout the animal kingdom up to human. At the centrosomes, Pcnt serves as a multifunctional scaffold for various proteins and protein complexes and has important functions in microtubule organization, cell division, cell cycle progression, assembly of cilia and probably in various other fundamental cellular processes.Citation1-Citation4 Pericentrin (PCNT) mutations are associated with various diseases, most prominent the Majewski/microcephalic osteodysplastic primordial dwarfism type II (MOPD II), a rare human autosomal recessive genetic disorder.Citation4-Citation6 The link between Pcnt and other diseases like human cancer, mental disorders and ciliopathies is not as strong as for MOPD II, but nevertheless cellular and molecular evidence supports a role for Pcnt in these disorders.Citation4 The localization of Pcnt at the base of primary cilia was shown a few years ago,Citation7 and since then Pcnt proved to play important roles in ciliary function.Citation7-Citation10 Only very recently, we showed the specific expression of a Pcnt splice variant at the basal body complex of the connecting cilium in photoreceptor cells of mice,Citation11 raising questions concerning the function of the Pcnt spliceome in general.
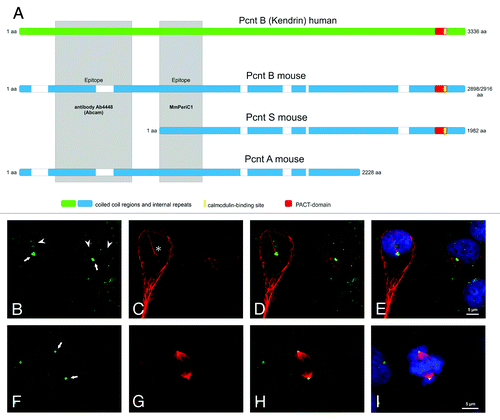
Pcnt is a large coiled-coil protein with so far three known splice variants from orthologous genes in mice and humans. The largest form, Pcnt B, has a molecular weight of ~360 kDa in mice and ~380 kDa in humans. Moreover, two smaller forms with a size of ~220–250 kDa, Pcnt A and Pcnt S, are known from mice ().Citation11-Citation15
Figure 1. Mouse and Human Pericentrin variants and Pericentrin distribution in human embryonic kidney cells. (A) Scheme of the known and published Pericentrin (Pcnt) splice variants: Pcnt B human (Kendrin, accession number (AN: NP_006022), Pcnt B mouse (Pcnt 360, AN: NP_032813 or BAF36559), Pcnt A mouse (AN: partial, AAO24322.1) and Pcnt S mouse (Pcnt 250, BAF36560). Human Pcnt is larger than mouse Pcnt—white bars in the mouse Pcnt variants indicate missing sequence parts. The homology between human and mouse Pcnt is about 60%. The epitopes of the affinity purified MmPeriC1 antibody and the Ab4448 antibody are indicated in gray. (B-I) Triple labeling of Pcnt (green, B and F), ac. tubulin (marker particularly for primary cilia, red, C and G), and DAPI (blue, E and I) in human embryonic kidney cells (HEK-293T cells). Pcnt is localized at the centrosomes of resting and dividing cells and at the basal body complex of primary cilia (arrows; primary cilium marked with an asterisk)). Moreover it accumulates in the nucleoli of interphase cells (arrowheads) and can be found distributed throughout the cytoplasm at granular appearing structures. (D, H) Merge of the Pcnt and ac. tubulin staining. (E, I) Merge of the Pcnt, ac. tubulin and DAPI staining. Scale bar: 5 µm (E, I).
Pcnt in the Mouse
In our study of the cellular expression and distribution of Pcnt in neuronal tissues of the mouse with a focus on the retina and its sensory neurons, the photoreceptors, we showed that Pcnt splice variants are differentially distributed in the examined tissues and even within the cells of one tissue.Citation11 The photoreceptors of mice contain predominantly one of the smaller Pcnt splice variants, most likely a modified variant of Pcnt S. In contrast, the larger Pcnt B splice variant is present in much lesser amounts in photoreceptors, but it is strongly expressed in other retinal cells or other neuronal tissues.Citation11 This specific distribution of the various Pcnt splice variants provides an explanation for the findings of Miyoshi and colleagues, who reported ciliary defects in the olfactory system of mice with a hypomorphic mutation in the PCNT gene in the region of exon 1, and the absence of a ciliary phenotype in other ciliated tissues like the retina with its photoreceptors.Citation10 The Pcnt splice variants present in the olfactory epithelium—Pcnt B and Pcnt ACitation11—both start with exon 1 and thus are affected by the hypomorphic mutation (). As mouse photoreceptors predominantly express Pcnt S, which is most likely still functional in the hypomorphic animals, the lack of a retinal phenotype is not astonishing.Citation11
We conclude that in the mouse different Pcnt splice variants are expressed in different tissues and even in different cells of one tissue. The consequence of such a patchwork of splice variants is that most mutations in the PCNT gene will not have global but tissue and even cell specific effects.
Pcnt in Human
In the last years, a lot of effort has been invested into deciphering the role of Pcnt in human. Diseases associated with mutations in the PCNT gene display heterogeneous clinical manifestations, making it difficult to pinpoint the functional role of Pcnt (for a review see ref. Citation4). The issue is further complicated by the fact that little is known about the expression of Pcnt splice variants in the various human tissues. Immunocytochemical stainings of human embryonic kidney cells (HEK-293T cells) with our affinity purified Pcnt antibody MmPeriC1, which should detect all splice variants that are known to date (), showed a localization of Pcnt at the centrosomes (see also refs. Citation1–Citation3, Citation11), at the basis of primary cilia (see also refs. Citation7, Citation8, Citation11), throughout the cytoplasm [probably at granular structures (see also refs. Citation16, Citation17)], and in the nucleoli of interphase cells (see also ref. Citation18) (). This wide distribution pattern most likely reflects the functional diversity of Pcnt and its splice variants in human cells. Based on what we know about the functional Pcnt patchwork in the mouse, we started to search for possible Pcnt splice variants in human tissues. We performed western blot experiments with our antibody MmPeriC1 using various human cell lines, i.e., HEK-293T cells, cervical cancer cells (HeLa cells), and two breast cancer cell lines, MCF-7 and MDA-MB 231 (). Mouse tissues and 3T3 mouse fibroblasts served as controls (). We found in the human cells, like in the mouse, different Pcnt positive bands with varying intensities on the protein level (). These findings corroborate earlier results from northern blot experiments, showing the existence of more than one Pcnt variant in humans.Citation12,Citation19
Figure 2. Expression of Pericentrin splice variants in different mouse and human tissues and cells. Every lane is loaded with approximately the same amount of protein. (A) Western blot analysis of mouse protein extracts of retina, olfactory epithelium and NIH 3T3 mouse fibroblasts using the MmPeriC1 antibody. A ~360 kDa protein band—mouse Pericentrin (Pcnt) B—is detected in all three samples. A second protein band with varying molecular weight—~250 kDa (most likely mouse Pcnt A and/or S) in olfactory epithelium and NIH 3T3 mouse fibroblasts, ~225 kDa (most likely a variant of mouse Pcnt S) in retina—suggests the existence of different Pcnt variants in different tissues, which are expressed at different protein levels. The third band in the olfactory epithelium at ~190 kDa might be a cleaved part of Pcnt, since it does not appear constantly in every experiment. (B) Western blot analysis of human protein extracts of HEK-293T cells (embryonic kidney), HeLa cells (cervical cancer), MCF-7 cells and MDA-MB 231 cells (both breast cancer) using the MmPeriC1 antibody. The ~380 kDa human Pcnt B is detected as a double band with different expression levels in the different cell lines. The constantly appearing double band might show a posttranslational modification of Pcnt B resulting in a weight shift. All four human cell extracts show a second and a third band with with molecular weights of ~270 kDa and 220 kDa (potentially human Pcnt A and S). In the three cancer cell lines an additional Pcnt positive band at ~200 kDa appears. All human cell lines show different expression levels of distinct bands, suggesting a unique expression pattern of different Pcnt splice variants in every human tissue. (C) Control western blot analysis of the human cell extracts used in B. Preadsorption of the MmPeriC1 antibody with the respective antigen in saturating concentrations blocks the detection of the protein bands in all human cell samples. For detailed experimental information see ref. Citation11.
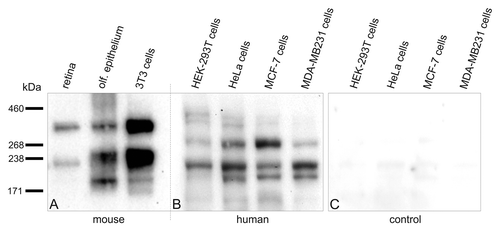
In MOPD II studies the loss of Pcnt positive bands in western blots of lymphoblastoid cells of patients was reported.Citation5,Citation14,Citation20 For the detection of Pcnt an antibody recognizing the N-terminal part of Pcnt (Ab4448, Abcam) was used. However, the use of such an antibody causes an experimental problem because it might not detect all Pcnt variants, e.g., Pcnt S or any other variants lacking the N-terminal region of Pcnt (). Indeed, using our antibody MmPeriC1, we find a different protein pattern in western blots of human tissues compared with the control cells in the MOPD II studies (). MOPD II patients show a severe and complex phenotype,Citation21,Citation22 but they are viable. As it is assumed for humans that a complete loss of Pcnt will lead to prenatal death,Citation23 and knockout mice with a complete loss of Pcnt are nonviable,Citation15 the question arises why the MOPD II phenotype is not lethal. There are only few possible explanations: The mutations in the PCNT gene are hypomorphic, or they are compensated for by the expression of other genes,Citation23 or, much simpler, not all splice variants of Pcnt are affected by the mutations.
Like in the mouse, our findings suggest also for humans a patchwork of different Pcnt splice variants in different tissues, which may explain why mutations in the human PCNT gene generate a multitude of different phenotypes.
For a final answer the Pcnt spliceome in human tissues has to be deciphered—a task that exceeds the possibilities of our group focusing on animal models. Nonetheless, we believe that the hypothesis of a functional Pcnt patchwork in humans is worth following up, as it will lead to a better understanding of disorders linked to PCNT mutations. In fact, this would be a good starting point for an interdisciplinary research project with basic and clinical research working hand in hand.
| Abbreviations: | ||
| Pcnt | = | Pericentrin (protein) |
| PCNT | = | Pericentrin (gene) |
| PCM | = | pericentriolar material |
| MOPD II | = | Majewski/microcephalic osteodysplastic primordial dwarfism type II |
Additional material
Download Zip (5.4 MB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (GI 770/1-1), the funds of the Dr. Hertha und Helmut Schmauser-Stiftung (Erlangen, Germany) and the Universitätsbund Erlangen-Nürnberg e.V. (Erlangen, Germany). The authors thank Beata Schmidt, Freya Boggasch and Nadja Schröder-Kress for skillful technical assistance and Prof. Dr. Johann Helmut Brandstätter for critical and helpful comments on the manuscript. We also thank Dr. Alexandra Schambony for providing us the MCF-7 and MDA-MB231 cells.
References
- Doxsey SJ, Stein P, Evans L, Calarco PD, Kirschner M. Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell 1994; 76:639 - 50; http://dx.doi.org/10.1016/0092-8674(94)90504-5; PMID: 8124707
- Takahashi M, Yamagiwa A, Nishimura T, Mukai H, Ono Y. Centrosomal proteins CG-NAP and kendrin provide microtubule nucleation sites by anchoring gamma-tubulin ring complex. Mol Biol Cell 2002; 13:3235 - 45; http://dx.doi.org/10.1091/mbc.E02-02-0112; PMID: 12221128
- Zimmerman WC, Sillibourne J, Rosa J, Doxsey SJ. Mitosis-specific anchoring of gamma tubulin complexes by pericentrin controls spindle organization and mitotic entry. Mol Biol Cell 2004; 15:3642 - 57; http://dx.doi.org/10.1091/mbc.E03-11-0796; PMID: 15146056
- Delaval B, Doxsey SJ. Pericentrin in cellular function and disease. J Cell Biol 2010; 188:181 - 90; http://dx.doi.org/10.1083/jcb.200908114; PMID: 19951897
- Rauch A, Thiel CT, Schindler D, Wick U, Crow YJ, Ekici AB, et al. Mutations in the pericentrin (PCNT) gene cause primordial dwarfism. Science 2008; 319:816 - 9; http://dx.doi.org/10.1126/science.1151174; PMID: 18174396
- Willems M, Geneviève D, Borck G, Baumann C, Baujat G, Bieth E, et al. Molecular analysis of pericentrin gene (PCNT) in a series of 24 Seckel/microcephalic osteodysplastic primordial dwarfism type II (MOPD II) families. J Med Genet 2010; 47:797 - 802; http://dx.doi.org/10.1136/jmg.2009.067298; PMID: 19643772
- Jurczyk A, Gromley A, Redick S, San Agustin J, Witman G, Pazour GJ, et al. Pericentrin forms a complex with intraflagellar transport proteins and polycystin-2 and is required for primary cilia assembly. J Cell Biol 2004; 166:637 - 43; http://dx.doi.org/10.1083/jcb.200405023; PMID: 15337773
- Miyoshi K, Onishi K, Asanuma MP, Miyazaki I, Diaz-Corrales FJ, Ogawa N. Embryonic expression of pericentrin suggests universal roles in ciliogenesis. Dev Genes Evol 2006; 216:537 - 42; http://dx.doi.org/10.1007/s00427-006-0065-8; PMID: 16534625
- Martinez-Campos M, Basto R, Baker J, Kernan M, Raff JW. The Drosophila pericentrin-like protein is essential for cilia/flagella function, but appears to be dispensable for mitosis. J Cell Biol 2004; 165:673 - 83; http://dx.doi.org/10.1083/jcb.200402130; PMID: 15184400
- Miyoshi K, Kasahara K, Miyazaki I, Shimizu S, Taniguchi M, Matsuzaki S, et al. Pericentrin, a centrosomal protein related to microcephalic primordial dwarfism, is required for olfactory cilia assembly in mice. FASEB J 2009; 23:3289 - 97; http://dx.doi.org/10.1096/fj.08-124420; PMID: 19470799
- Mühlhans J, Brandstätter JH, Gießl A. The centrosomal protein pericentrin identified at the basal body complex of the connecting cilium in mouse photoreceptors. PLoS One 2011; 6:e26496; http://dx.doi.org/10.1371/journal.pone.0026496; PMID: 22031837
- Flory MR, Davis TN. The centrosomal proteins pericentrin and kendrin are encoded by alternatively spliced products of one gene. Genomics 2003; 82:401 - 5; http://dx.doi.org/10.1016/S0888-7543(03)00119-8; PMID: 12906865
- Miyoshi K, Asanuma M, Miyazaki I, Matsuzaki S, Tohyama M, Ogawa N. Characterization of pericentrin isoforms in vivo. Biochem Biophys Res Commun 2006; 351:745 - 9; http://dx.doi.org/10.1016/j.bbrc.2006.10.101; PMID: 17084386
- Piane M, Della Monica M, Piatelli G, Lulli P, Lonardo F, Chessa L, et al. Majewski osteodysplastic primordial dwarfism type II (MOPD II) syndrome previously diagnosed as Seckel syndrome: report of a novel mutation of the PCNT gene. Am J Med Genet A 2009; 149A:2452 - 6; http://dx.doi.org/10.1002/ajmg.a.33035; PMID: 19839044
- Endoh-Yamagami S, Karkar KM, May SR, Cobos I, Thwin MT, Long JE, et al. A mutation in the pericentrin gene causes abnormal interneuron migration to the olfactory bulb in mice. Dev Biol 2010; 340:41 - 53; http://dx.doi.org/10.1016/j.ydbio.2010.01.017; PMID: 20096683
- Kubo A, Tsukita S. Non-membranous granular organelle consisting of PCM-1: subcellular distribution and cell-cycle-dependent assembly/disassembly. J Cell Sci 2003; 116:919 - 28; http://dx.doi.org/10.1242/jcs.00282; PMID: 12571289
- Jurczyk A, Pino SC, O’Sullivan-Murphy B, Addorio M, Lidstone EA, Diiorio P, et al. A novel role for the centrosomal protein, pericentrin, in regulation of insulin secretory vesicle docking in mouse pancreatic beta-cells. PLoS One 2010; 5:e11812; http://dx.doi.org/10.1371/journal.pone.0011812; PMID: 20676397
- Charters GA, Stones CJ, Shelling AN, Baguley BC, Finlay GJ. Centrosomal dysregulation in human metastatic melanoma cell lines. Cancer Genet 2011; 204:477 - 85; http://dx.doi.org/10.1016/j.cancergen.2011.07.001; PMID: 22018269
- Flory MR, Moser MJ, Monnat RJ Jr., Davis TN. Identification of a human centrosomal calmodulin-binding protein that shares homology with pericentrin. Proc Natl Acad Sci U S A 2000; 97:5919 - 23; http://dx.doi.org/10.1073/pnas.97.11.5919; PMID: 10823944
- Griffith E, Walker S, Martin CA, Vagnarelli P, Stiff T, Vernay B, et al. Mutations in pericentrin cause Seckel syndrome with defective ATR-dependent DNA damage signaling. Nat Genet 2008; 40:232 - 6; http://dx.doi.org/10.1038/ng.2007.80; PMID: 18157127
- Hall JG, Flora C, Scott CI Jr., Pauli RM, Tanaka KI. Majewski osteodysplastic primordial dwarfism type II (MOPD II): natural history and clinical findings. Am J Med Genet A 2004; 130A:55 - 72; http://dx.doi.org/10.1002/ajmg.a.30203; PMID: 15368497
- Brancati F, Castori M, Mingarelli R, Dallapiccola B. Majewski osteodysplastic primordial dwarfism type II (MOPD II) complicated by stroke: clinical report and review of cerebral vascular anomalies. Am J Med Genet A 2005; 139:212 - 5; http://dx.doi.org/10.1002/ajmg.a.31009; PMID: 16278902
- Delaval B, Doxsey S. Genetics. Dwarfism, where pericentrin gains stature. Science 2008; 319:732 - 3; http://dx.doi.org/10.1126/science.1154513; PMID: 18258883