Abstract
Wiskott-Aldrich Syndrome protein (WASp) is an actin nucleation-promoting factor that regulates actin polymerisation via the Arp2/3 complex. Its mutation in human syndromes has led to extensive studies on the regulation and activities of this molecule. Several mechanisms for the regulation of WASp activity have been proposed, however, the role of tyrosine phosphorylation remains controversial, particularly due to inconsistencies between results obtained through biochemical and cell biological approaches. In this mini-review, we are addressing the major aspects of WASp regulation with an emphasis on the role of tyrosine phosphorylation on WASp activities.
Introduction
WASp is a haematopoietic-specific protein involved in actindependent events, such as cell motility, phagocytosis and immune synapse formation. Mutations associated with decreased WASp activity have been associated with Wiskott-Aldrich syndrome and X-linked thrombocytopenia, while activating mutations have been implicated in severe congenital neutropenia. Therefore, understanding the precise mechanisms by which WASp activity is regulated is clinically relevant as constitutive activation or inactivation of WASp result in phenotypically distinct diseases. Non-haematopoietic cells express neural (N)-WASP, which shows identical domain organisation and high primary sequence homology to WASp, even though several regions of divergence exist between the two molecules. Interestingly, several haematopoietic cells (e.g., platelets, monocytic lineage cells) express both WASp and N-WASP, and to what extend they may have unique or redundant functions remains unknown.
Structure-Function Relationship: Autoinhibition, Recruitment and Activation of WASp
WASp is a multidomain protein comprised, from the N to the C terminus, of a WH1 domain, followed by a basic domain, a G protein binding (GBD) domain, a proline-rich region and a VCA domain. These domains contribute to protein-lipid and protein-protein interactions important for the localization and activation of WASp, and its ubiquitously expressed homologue, N-WASP. Several reviews have explored these interactions in great detail and the readers are referred to these for more information.Citation1–Citation3 Briefly, WASp (and N-WASP) is autoinhibited by a set of intramolecular interactions mainly between the basic and GBD domains with the VCA domain, thus preventing binding of the Arp2/3 to the VCA domain and initiation of actin polymerization ().
The first mechanism of activation to be identified involved the discovery that WASp is an effector of the small GTPase Cdc42, and subsequently that phosphatidyl inositol 4,5-bisphosphate (PtdIns(4,5)P2) acts co-operatively with Cdc42 for WASp activation.Citation4–Citation7 We have also confirmed the dependence of Cdc42 in WASp activation through dominant negative Cdc42 overexpression or shRNA-mediated downregulation of endogenous Cdc42 levels, and the use of a fluorescence resonance energy transfer (FRET)-based biosensor and a conformation sensitive antibody to report the conformation state of WASp in macrophages stimulated with CSF-1.Citation8 Both approaches indicated that WASp was folded, and consequently inactive, under conditions where Cdc42 was inhibited. Interestingly, recruitment of WASp to sites of activity, such as regions of T cell receptor (TCR) engagementCitation9 or the phagosome in macrophages,Citation10 occurs independently of Cdc42 and PtdIns(4,5)P2 and depends on the proline-rich region of WASp. This points to a role of SH3 domain containing proteins in the recruitment of WASp in vivo. One such protein is Nck, which can also activate WASp independently of Cdc42, via its three SH3 domains, and can co-operate with PtdIns(4,5)P2 for enhanced actin polymerization in vitro.Citation11 Inducible clustering of Nck SH3 domains can also stimulate N-WASP dependent actin polymerization in cells.Citation12 Recently it was shown that the multiple SH3 domains of Nck are required for dimerisation of WASp; WASp dimers can bind the Arp2/3 complex with higher affinity, thus enhancing actin polymerisation.Citation11 Similar mechanisms of WASp di- or poly-merisation, though independent of polyproline region—SH3 domain interactions, may also be employed by the bacterial protein EspFu to promote motility of bacterial pathogens infecting cells.Citation11,Citation13–Citation15
Regulation of WASp by phosphorylation.
Both serine and tyrosine phosphorylation of WASp have been reported to take place in two distinct sites on the WASp molecule; serines 483 and 484 in the VCA domain (at the junction between the V and A regions),Citation16 and serine 277,Citation17 and tyrosine 291,Citation18 in the GBD domain. The corresponding phosphorylation sites are also conserved in N-WASP, for which a separate tyrosine residue, Y175,Citation19 has also been shown to be phosphorylated, albeit to a lesser extent, and its contribution to N-WASP activity and/or actin polymerization has not been assessed.
S483/484 phosphorylation, mediated by casein kinase II, is constitutive and perhaps associated with the maturation of competent species in vivo.Citation16 Indeed, phosphorylation of these two serine residues increases affinity of WASp for Arp3, and is required for efficient actin polymerization by the full-length WASp in vitro and in vivo.Citation16 Y291 on the other hand, is phosphorylated in a regulated manner and several stimuli have been shown to increase phospho-Y291 in cells, and a range of kinases has been proposed to phosphorylate WASp/N-WASP in vitro and in vivo (). These include Src family kinases, FAK, ACK1, Btk and potentially Syk. S277 was phosphorylated by the dual kinase ACK1, along with Y291, and phosphorylation of this site contributed to increased actin polymerization in vitro, though its relevance in intact cells remains unexplored.Citation17
The effect of Y291 phosphorylation on WASp (and the equivalent Y253 in N-WASP) activity has been extensively studied in vitro using mainly actin pyrene assays as the output of activity. Collectively, these studies have uncovered a model whereby unfolding of WASp by Cdc42 binding activates WASp and exposes Y291 for phosphorylation. This results in enhanced Arp2/3-dependent actin polymerization as the affinity of the GBD domain towards the VCA domain is significantly reduced after phosphorylation thus favouring VCA-Arp2/3 interactions.Citation20,Citation21 Additionally, phosphorylation of WASp primes the molecule to activation by SH2 and/or tandem SH2-SH3 domains, such as those of Src family kinases, when Cdc42 is no longer binding. The SH2 domain of Src was reported to bind phosphorylated GBD with considerable affinity (Kd of ∼2 µM) and disrupt the GBD interaction with the VCA domain. This is further supported by the fact that the autoinhibited phospho-WASp is less susceptible to dephosphorylation by phosphatases.Citation20,Citation21 These in vitro studies suggest that tyrosine phosphorylation of WASp enhances WASp/N-WASP actin polymerization activity ().
These elegant biochemical studies have shed light in the role of phosphorylation whereby actin polymerization is the direct output of WASp activity. However the role of WASp phosphorylation in cells remains relatively unknown.
Regulation of phosphorylation in vivo.
summarizes the range of signals that can result in WASp phosphorylation in cells. Consistent with in vitro studies, Cdc42-independent activation and phosphorylation of WASp by Fyn results in actin polymerization following TCR ligation.Citation22 However, this may be a result of a combination of SH3 domain and PtdIns(4,5)P2 interactions driving WASp targeting and unfolding prior to phosphorylation. Our data using Cdc42 binding deficient WASp mutants or Cdc42-silenced macrophages, indicates a role for Cdc42 in inducing WASp phosphorylation by pervanadate treatmentCitation8 and during Fcγ receptor (FcγR)-mediated phagocytosis,Citation23 supporting the model by Torres and Rosen,Citation20,Citation21,Citation24 whereby Cdc42 provides conformational relief of autoinhibition and accessibility of the GBD to kinases. Furthermore, overexpression of the activating mutation L270P, identified in patients with severe congenital neutropenia that stabilizes WASp in an open conformation,Citation25 resulted in high basal tyrosine phosphorylation of WASp.Citation23 Also, the open conformation was able to overcome the effect of a Cdc42 binding abolishing mutation in pervanadate-induced tyrosine phosphorylation.Citation8,Citation23 It remains unknown, however, whether the L270P mutation, or indeed the other activating mutations, L272P and I294T,Citation26 results in high basal phosphorylation of WASp in cells of patients with severe congenital neutropenia and whether this may contribute to the pathology of this disease.
Effects of phosphorylation in cellular processes and in vivo.
Most studies addressing the role WASp phosphorylation plays in cellular functions have utilized primarily overexpression of WASp bearing tyrosine mutations to either mimic or abolish phosphorylation, with results either consistent or inconsistent with in vitro observations. For example, overexpression of Y291E WASp in macrophages resulted in increased filopodia formation,Citation27 Y253E N-WASP promoted neurite extension,Citation28 while over-expression of Y291F WASp in T cells resulted in decreased actin polymerization downstream of TCR engagement.Citation22 Our data examining podosome dynamics in macrophages suggest a faster rate of podosome assembly in the Y291E-expressing cells and a higher F-actin content per podosome area, while they also exhibit increased resistance to disassembly by wiskostatin,Citation29 a compound that inhibits WASp (and N-WASP) activity by stabilizing the autoinhibited conformation.Citation30 These observations are consistent with increased activity of phosphorylated WASp in cells.
Interestingly, our data using a FRET-based WASp biosensor indicated that WASp conformation under basal conditions and in the podosomes of macrophages was similar between the Y291E and Y291F biosensor mutants.Citation8,Citation29 Changes in conformation may, as a result, not necessarily indicate an active species. It is, therefore, possible that WASp phosphorylation in vivo may not be exclusively associated with actin polymerization but may also influence the stability of actin filaments through decrease in depolymerisation. Accordingly, F-actin in macrophage podosomes regulated by Y291E WASp is more stable than in those regulated by Y291F WASp, even though both mutants result in apparent equal activity as evidenced by FRET.Citation29
An important drawback in the use of the Y291E phosphomimetic, however, is that it may be insufficient in mimicking phosphorylation, while additionally it excludes the input of SH2 domain binding. This could explain why in some cases the Y291E mutation does not enhance actin-dependent events in vivo (e.g., actin tail length beneath Shigella pathogensCitation31) as opposed to in vitro settings. Furthermore, it may also hinder processes whereby phosphorylation/dephosphorylation may be required for an obvious cellular phenotype, as our data have uncovered in the chemotaxis of macrophages to CSF-1.Citation29
Additionally, our group and others have shown that FcγR-mediated phagocytosis in macrophages requires WASp Y291 phosphorylation.Citation23,Citation32 Importantly, our group has shown that overexpression of the constitutively active L270P WASp mutant, when coupled to the Y291F phospho-abolishing mutation, decreased phagocytosis in control macrophages.Citation23 Furthermore, in Cdc42-silenced macrophages, the phagocytosis defect associated with loss of endogenous Cdc42 could be partially rescued by the activated L270P WASp mutant, but when the L270P mutation was coupled to the Y291F mutation, phagocytosis remained unaltered.Citation23 This indicates that phosphorylation plays additional roles other than simply shifting the balance towards the open conformation of WASp, and one speculation is that it may regulate the interaction with a SH2 domain containing protein. Consistently, the Y291E mutation only partially rescued phagocytosis in this setting.
The importance of WASp phosphorylation in vivo was recently highlighted in a study using mouse knock-ins of WASp phosphomutants (Y293E/F, the equivalent of Y291 in murine WASp), showing a requirement for tyrosine phosphorylation in several aspects of T cell development and cellularity in vivo, as well as red blood cell count.Citation33 Therefore, WASp phosphorylation is required for events regulating immune system maturation in whole organisms, since in several aspects of leukocyte development the Y293F WASp knock-in mice were similar to the WASp knock-out ones.
The Role of Tyrosine Phosphorylation in WASp Turnover
Several studies have also demonstrated that instead of an activating signal, tyrosine phosphorylation may result in the proteolytic degradation of WASp/N-WASP, consequently resulting in inactivation. For example, in platelets, collagen-induced WASp phosphorylation was too transient to account for the “priming” model described above. Instead, phosphorylation of endogenous WASp was coupled to an almost instantaneous calpain-mediated cleavage of the membrane-associated WASp and clear accumulation of proteolytic fragments.Citation34 Furthermore, under certain experimental conditions, expression of Y253E N-WASP in neurons resulted in its ubiquitination and proteasome-mediated degradation.Citation28 Additionally, a recent study demonstrated that in dendritic cells isolated from transgenic mice with a WASp Y293E knock-in, WASp protein levels were significantly reduced and that this decrease could be partially rescued by proteasome but not calpain inhibitors.Citation33 Interestingly, N-WASP was found to be insensitive to calpain proteolysis in blood cells that express both WASp and N-WASP.Citation35 Phosphorylation may therefore occur transiently following WASp activation, subsequently acting as an inactivation signal through degradation pathways. It is interesting however, to note that the L270P mutation has not been reported to result in proteolytic degradation of WASp even though it results in an unfolded molecule with high basal phosphorylation levels.Citation23 Potentially, the constitutively open conformation favours stable interactions with molecules that could prevent degradation, consequently prolonging its activity in cells.
The Role of Phosphorylation in WASp Subcellular Localization
The localization of WASp may also be regulated by Y291 phosphorylation. A striking example is the complete loss of nuclear localization of the Y253D phosphomimetic N-WASP mutant when transfected in NIH3T3 cells compared to wild-type and Y253F N-WASP.Citation36 A more subtle change could be observed in the localization of the unfolded WASp species in macrophages.Citation29 Through the use of a FRET WASp biosensor, coupled to TIRF microscopy, our group was able to observe highly restricted localization of the unfolded wild-type WASp in macrophage podosomes but not other areas in contact with the substrate. In contrast, the unfolded Y291F biosensor was aberrantly localized throughout the ventral surface of the cell. This difference could not be observed by other means of immunofluorescence as they cannot distinguish the active species. While there was no overall change in the distribution of F-actin, this localization defect of unfolded Y291F WASp could explain the less stable actin filaments in podosomes of macrophages expressing the Y291F mutant.Citation29
Conclusions
The many levels of WASp regulation highlight the importance of tightly controlling WASp activity in leukocytes. Tyrosine phosphorylation of WASp has been demonstrated in many cases, however, its precise functions in vivo remain unknown primarily because the use of glutamic acid substitutions may not completely mimic phosphorylation and prevent potential interactions with SH2 domain—containing proteins. Nevertheless, the extensive studies performed on patients with genetic syndromes and transgenic mice continue to shed light on this important molecule.
Abbreviations
| WASp | = | wiskott-aldrich syndrome protein |
| N-WASP | = | neural WASP |
| PtdIns(4,5)P2 | = | phosphatidyl inositol 4,5-bisphosphate |
| GBD | = | G protein binding domain |
| FRET | = | fluorescence resonance energy transfer |
| TCR | = | T cell receptor |
Figures and Tables
Figure 1 A model for the regulation of WASp by phosphorylation. The folded, inactive WASp molecule can be recruited to areas of receptor signalling via SH3 domain-containing adaptors (e.g., Nck) (A), were it can be activated by Cdc42 and PtdIns(4,5)P2 and phosphorylated by a kinase, such as Src (B). Once Cdc42 activity is terminated, the phosphorylated species (C) may be dephosphorylated by a phosphatase and go through further rounds of activation (A) or it may be proteolytically degraded (D). Alternatively, the phosphorylated species, may, in the absence of Cdc42 binding, refold and thus be “primed” (E) for activation by signals other than Cdc42, such as binding by SH2- and SH3 domain—containing proteins, and thus become active once again (F).
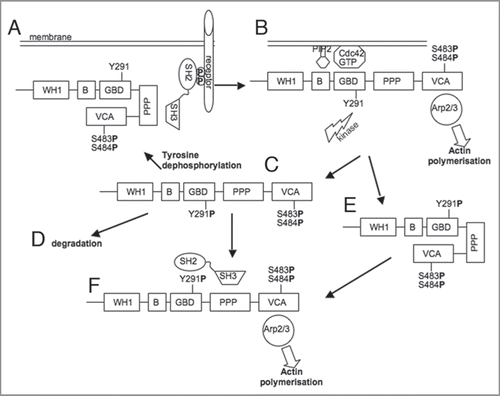
Table 1 Stimuli inducing WASp phosphorylation in cells
Acknowledgements
Work in the laboratory of Dianne Cox is supported by NIH grant GM071828.
References
- Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol 2007; 8:37 - 48
- Kurisu S, Takenawa T. The WASP and WAVE family proteins. Genome Biol 2009; 10:226
- Pollitt A, Insall RH. WASP and SCAR/WAVE proteins: the drivers of actin assembly. J Cell Sci 2009; 122:2575 - 2578
- Symons M, Derry JMJ, Karlak B, Jiang S, Lemahieu V, McCormick F, et al. Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization. Cell 1996; 84:723 - 734
- Aspenström P, Lindberg U, Hall A. Two GTPases, Cdc42 and Rac, bind directly to a protein implicated in the immunodeficiency disorder Wiskott-Aldrich syndrome. Curr Biol 1996; 6:70 - 75
- Higgs HN, Pollard TD. Activation by Cdc42 and PIP2 of Wiskott-Aldrich Syndrome protein (WASp) stimulates actin nucleation by Arp2/3 complex. J Cell Biol 2000; 150:1311 - 1320
- Rohatgi R, Ho HY, Kirschner MW. Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4,5-bisphosphate. J Cell Biol 2000; 150:1299 - 1310
- Cammer M, Gevrey J-C, Lorenz M, Dovas A, Condeelis J, Cox D. The mechanism of CSF-1 induced wasp activation in vivo: A role for PI3-kinase and CDC42. J Biol Chem 2009; 284:23302 - 23311
- Cannon JL, Labno CM, Bosco G, Seth A, McGavin MHK, Siminovitch KA, et al. WASP recruitment to the T cell:APC contact site pccurs independently of Cdc42 activation. Immunity 2001; 15:249 - 259
- Mao YS, Yamaga M, Zhu X, Wei Y, Sun H-Q, Wang J, et al. Essential and unique roles of PIP5K-γ and -α in Fcγ receptor-mediated phagocytosis. J Cell Biol 2 2009; 184:281 - 296
- Padrick SB, Cheng HC, Ismail AM, Panchal SC, Doolittle LK, Kim S, et al. Hierarcical regulation of WASP/WAVE proteins. Mol Cell 2008; 32:426 - 438
- Rivera GM, Briceño CA, Takeshima F, Snapper SB, Mayer BJ. Inducible clustering of membrane-tageted SH3 domains of the adaptor protein Nck triggers localized actin polymerization. Curr Biol 2 2004; 14:11 - 22
- Cheng H-C, Skehan BM, Campellone KG, Leong JM, Rosen MK. Structural mechanism of WASP activation by the enterohaemorrhagic E. coli effector EspFu. Nature 2008; 454:1009 - 1014
- Campellone KG, Cheng H-C, Robbins D, Siripala AD, McGhie EJ, Hayward RD, et al. Repetitive N-WASP-binding elements of the enterohemorrhagic Escherichia coli effector EspFu synergistically activate actin assembly. PLoS Pathog 2008; 4:10000191
- Sallee NA, Rivera GM, Dueber JE, Vasilescu D, Mullins RD, Mayer BJ, Lim WA. The pathogen protein EspFu hijacks actin polymerization using mimicry and multivalency. Nature 2008; 454:1005 - 1008
- Cory GOC, Cramer R, Blancholn L, Ridley AJ. Phosphorylation of the WASP-VCA domain increases its affinity for the Arp2/3 complex and enhances actin polymerization by WASP. Mol Cell 2003; 11:1229 - 1239
- Yokoyama N, Lougheed J, Miller WT. Phosphorylation of WASP by the Cdc42-associated Kinase ACK1. DUAL HYDROXYAMINO ACID SPECIFICITY IN A TYROSINE KINASE. J Biol Chem 2005; 280:42219 - 42226
- Baba Y, Nonoyama S, Matsushita M, Yamadori T, Hashimoto S, Imai K, et al. Involvement of wiskottaldrich syndrome protein in B-cell cytoplasmic tyrosine kinase pathway. Blood 1999; 93:2003 - 2012
- Burton EA, Oliver TN, Pendergast AM. Abl kinases regulate actin comet tail elongation via an N-WASP-dependent pathway. Mol Cell Biol 2005; 25:8834 - 8843
- Torres E, Rosen MK. Contingent phosphorylation/dephosphorylation provides a mechanism of molecular memory in WASP. Mol Cell 2003; 11:1215 - 1227
- Torres E, Rosen MK. Protein-tyrosine kinase and GTPase signals cooperate to phosphorylate and activate Wiskott-Aldrich Syndrome Protein (WASP)/Neuronal WASP. J Biol Chem 2006; 281:3513 - 3520
- Badour K, Zhang J, Shi F, Leng Y, Collins M, Siminovitch KA. Fyn and PTP-PEST-mediated regulation of Wiskott-Aldrich Syndrome Protein (WASp) tyrosine phosphorylation is required for coupling T cell antigen receptor engagement to WASp effector function and T cell activation. J Exp Med 2004; 199:99 - 111
- Park H, Cox D. Cdc42 Regulates Fc? receptor-mediated phagocytosis through the activation and phosphorylation of Wiskott-Aldrich Syndrome Protein (WASP) and Neural-WASP. Mol Biol Cell 2009; 20:4500 - 4508
- Caron E. Regulation by phopshorylation: yet another twist in the WASP story. Dev Cell 2003; 4:772 - 773
- Devriendt K, Kim AS, Mathijs G, Frints SGM, Schwartz M, Van den Oord JJ, et al. Constitutively activating mutation in WASP causes X-linked severe congenital neutropenia. Nat Gen 2001; 27:313 - 317
- Ancliff PJ, Blundell MP, Cory GO, Calle Y, Worth A, Kempski H, et al. Two novel activating mutations in the Wiskott-Aldrich syndrome protein result in congenital neutropenia. Blood 2006; 108:2182 - 2189
- Cory GOC, Garg R, Cramer R, Ridley AJ. Phosphorylation of tyrosine 291 enhances the ability of WASp to stimulate actin polymerization and filopodium formation. J Biol Chem 2002; 277:45115 - 45121
- Suetsugu S, Hattori M, Miki H, Tezuka T, Yamamoto T, Mikoshiba K, Takenawa T. Sustained activation of N-WASP through phosphorylation is essential for neurite extension. Dev Cell 2002; 3:645 - 658
- Dovas A, Gevrey J-C, Grossi A, Park H, Abou-Kheir W, Cox D. Regulation of podosome dynamics by WASp phosphorylation: implication in matrix degradation and chemotaxis in macrophages. J Cell Sci 2009; 122:3873 - 3882
- Peterson JR, Bickford LC, Morgan D, Kim AS, Ouerfelli O, Kirschner MW, Rosen MK. Chemical inhibition of N-WASP by stabilization of a native autoinhibited conformation. Nat Struct Mol Biol 2004; 11:747 - 755
- Adamovich DA, Nakamura F, Worth A, Burns S, Thrasher AJ, Hartwig JH, Snapper SB. Activating mutations of N-WASP alter Shigella pathogenesis. Biochem Biophys Res Commun 2009; 384:284 - 289
- Tsuboi S, Meerloo J. Wiskott-Aldrich Syndrome protein is a key regulator of the phagocytic cup formation in macrophages. J Biol Chem 2007; 282:34194 - 34203
- Blundell MP, Bouma G, Metelo J, Worth A, Calle Y, Cowell LA, et al. Phosphorylation of WASP is a key regulator of activity and stability in vivo. Proc Natl Acad Sci USA 2009; 106:15738 - 15743
- Lutskiy MI, Shcherbina A, Bachli ET, Cooley J, Remold-O’Donnell E. WASP localizes to the membrane skeleton of platelets. Br J Haematol 2007; 139:98 - 105
- Shcherbina A, Miki H, Kenney DM, Rosen FS, Takenawa T, Remold-O’Donnell E. WASP and N-WASP in human platelets differ in sensitivity to protease calpain. Blood 2001; 98:2988 - 2991
- Wu X, Suetsugu S, Cooper LA, Takenawa T, Guan JL. Focal adhesion kinase regulation of N-WASP subcellular localization and function. J Biol Chem 2004; 279:9565 - 9576
- Oda A, Ochs H, Druker BJ, Ozaki K, Watanabe C, Handa M, et al. Collagen induces tyrosine phosphorylation of Wiskott-Aldrych Syndrome protein in human platelets. Blood 1998; 92:1852 - 1858
- Gismondi A, Cifaldi L, Mazza C, Giliani S, Parolini S, Morrone S, et al. Impaired natural and CD16-mediated NK cell cytotoxicity in patients with WAS and XLT: ability of IL-2 to correct NK cell functional defect. Blood 2004; 104:436 - 443
- Guinamard R, Aspenström P, Fougereau M, Chavrier P, Guillemot J-C. Tyrosine phosphorylation of the Wiskott-Aldrich Syndrome protein by Lyn and Btk is regulated by CDC42. FEBS Lett 1998; 434:431 - 436
- Soriani A, Moran B, De Virgilio M, Kawakami T, Altman A, Lowell C, et al. A role for PKCϑ in outside-in αIIbβ3 signaling. J Thromb Hemost 20 2006; 4:648 - 655
- Oda A, Ochs H, Lasky LA, Spencer S, Ozaki K, Fujihara M, et al. CrkL is an adapter for Wiskott-Aldrich syndrome protein. Blood 2001; 97:2633 - 2639
- Gross BS, Wilde JI, Quek L, Chapel H, Nelson DL, Watson SP. Regulation and Function of WASp in Platelets by the Collagen Receptor, Glycoprotein VI. Blood 1999; 94:4166 - 4176
- Chellaiah MA, Kuppuswamy D, Lasky L, Linder S. Phosphorylation of a Wiscott-Aldrich Syndrome Protein-associated signal complex is critical in osteoclast bone resorption. J Biol Chem 2007; 282:10104 - 10116