Abstract
Elucidation of in vivo cholesterol transport and its aberrations in cardiovascular diseases requires suitable model organisms and the development of appropriate monitoring technology. We presented recently a new approach to visualize transport of the intrinsically fluorescent sterol, dehydroergosterol (DHE) in the genetically tractable model organism Caenorhabditis elegans (C. elegans). DHE is structurally very similar to cholesterol and ergosterol, two sterols used by the sterol-auxotroph nematode. We developed a new computational method measuring fluorophore bleaching kinetics at every pixel position, which can be used as a fingerprint to distinguish rapidly bleaching DHE from slowly bleaching autofluorescence in the animals. Here, we introduce multicolour bleach-rate sterol imaging. By this method, we demonstrate that some DHE is targeted to a population of basolateral recycling endosomes (RE) labelled with GFP-tagged RME-1 (GFP-RME- 1) in the intestine of both, wild-type nematodes and mutant animals lacking intestinal gut granules (glo1-mutants). DHE-enriched intestinal organelles of glo1-mutants were decorated with GFPrme8, a marker for early endosomes. No co-localization was found with a lysosomal marker, GFPLMP1. Our new methods hold great promise for further studies on endosomal sterol transport in C. elegans.
Introduction
Atherosclerosis and related cardiovascular diseases are the foremost causes of death and disability in the western society. These diseases have a complex etiology and involve many physiological processes. One of the risk factors is high blood cholesterol; specifically that linked to low density lipoprotein (LDL). Large research efforts has been made and knowledge about inter-organ transport of cholesterol in humans grew tremendously over the last decade. However, our understanding of intracellular cholesterol transport, its genetic regulation and coordination between various tissues is rather sparse. A classical approach for investigation of intracellular cholesterol transport is to choose first an appropriate cell model for typical sterol regulating tissues, like hepatocytes, smooth muscle cells, macrophages or adipocytes. Often radioactive cholesterol precursors, the cholesterol-binding filipin or suitable fluorescent sterols are used to monitor intercompartment sterol trafficking in these cell models.Citation1 A large body of data has been collected and important insight has been obtained by this approach. However, the explanatory power of this line of research is limited to the function of single cells, and extrapolation to cholesterol transport in complex 3D-tissues is difficult. Another strategy is to study sterol transport on the level of an animal with similar physiology and genetic regulation as humans, traditionally in mice, for example to investigate reverse cholesterol transport from peripheral cells to the liver.Citation2
We decided to follow a middle course between the cellular level and the mammalian animal model by investigating transport of a fluorescent sterol in the genetic model organism Caenorhabditis elegans (C. elegans). C. elegans is auxotroph for sterols and has been proven to be a great model for exploring the genetics of fat storage and lipid metabolism.Citation3 It is easy to handle in genetic experiments using RNA interference (RNAi). C. elegans has an additional, very interesting property being of high importance for the general understanding of the function of cholesterol and related sterols in cell biology: the nematodes need only spurious amounts of sterols in their food and can chemically modify the ingested sterols. They synthesize 4α-methylated sterols and steroid hormones, the latter to regulate the larval development of the worms.Citation4 Thus, C. elegans does not need cholesterol or ergosterol as structural component of all cellular membranes, which is in stark contrast to mammalian cells.Citation1,Citation4 To determine the tissue distribution and transport of sterols, Kurzchalia, Maxfield and colleagues introduced UV-sensitive wide field microscopy of the intrinsically fluorescent sterol dehydroergosterol (DHE) in C. elegans.Citation5 Their study provided important new insight into the selective enrichment of a sterol in selected tissues, but it suffered from an unsatisfactory discrimination between probe fluorescence (DHE) and cellular autofluorescence. DHE is structurally closely related to the two sterols used by the nematodes, cholesterol and ergosterol (see ).
Since autofluorescence of gut granules and other structures in C. elegans spectrally overlap largely with emission of DHE,Citation6 intensity-based methods will not be able to sufficiently discriminate the sterol from autofluorescence. We developed a new computational imaging method to overcome this problemCitation7 Pixel-wise analysis of photobleaching of all fluorescence of the nematodes in the UV region of the spectrum (between 370–420 nm) revealed that DHE bleaches at least fivefold faster than autofluorescence in all tissues of the worms. By fitting a monoexponential decay function to the fading intensity, we were able to use the estimated bleach time constant as fingerprint to detect DHE in the presence of high autofluorescence (). Our software ‘PixBleach’ is freely available as plugin to the popular image analysis program, ImageJ (rsbweb.nih.gov/ij/) at bigwww.epfl.ch/algorithms/pixbleach.Citation7 It provides spatial maps of bleach time constants, amplitude of the bleaching fraction and background image, in case of a monoexponential model; but it is programmed to allow for ease extension to other bleaching models, for example a stretched exponential function (). In addition, PixBleach calculates the root mean square error between data and model and the Chi-square values.Citation7 A recent extension of PixBleach allows even for a pixel-wise correction of the photobleaching process and shows that the fitted amplitude image is of significantly higher quality than the raw data.Citation8 Using bleach-rate-based image segmentation, we compared sterol trafficking in wild-type nematodes and in worms lacking intestinal lysosomal-like granules (gut granule loss, glo-phenotype).Citation7,Citation9 Glomutant animals lack birefringent and autofluorescent gut granules and expel the lysosomal content into the intestinal lumen.Citation9 We found a similar distribution of DHE in wild-type and glo-animals, but with strongly increased sterol enrichment in the intestine of the latter. Other tissues containing significant amounts of DHE were the reproductive organs and neurons in the head region.Citation7 The fact, that we find DHE enriched in certain tissues indicates, that in some cell types sterols might be of relevance for maintenance of integrity and function of cellular membranes in the nematodes, for example in intestinal epithelial cells and in neurons.
To characterize sterol containing tissue and organelles further requires co-staining with suitable markers. Recently, a screen for endocytic mutants in C. elegans has revealed several key molecules involved in receptor-mediated enodcytosis (RME).Citation10 It was shown that RME-1 is a protein involved in endocytic recycling of receptors like the transferrin receptor (Tf-R) in the intestine and in oocytes.Citation11 Subsequent studies in mammalian cells expressing RME-1 demonstrated that this EHD protein regulates receptor recycling from the endocytic recycling compartment (ERC), a type of recycling endosome (RE).Citation12 The ERC was found to be a major sterol pool in mammalian cells, and recycling of DHE from the ERC to the cell surface was found to require functional RME-1 in Chinese hamster ovarian cells.Citation13 Similarly, REs of polarized epithelial mammalian cells were found to contain significant amounts of cholesterol,Citation14 or-after pulselabeling of the plasma membrane-to receive large amounts of DHE.Citation15,Citation16
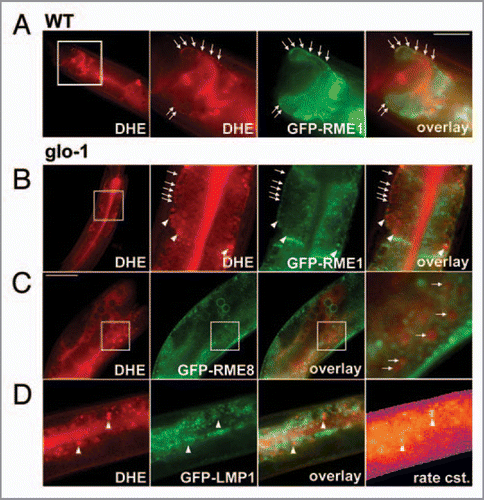
By feeding nematodes expressing a green fluorescent protein-tagged RME-1 (GFP-RME-1) with DHE, we observed in both, the wild-type and glo1-mutant animals co-localization of the sterol with GFP-RME-1 in REs close to the basolateral membrane of intestinal cells (, arrows). More apical located GFPRME1-positive endosomes in the intestine were not labeled with DHE. In glo-1 mutant animals lacking a functional rab GTPase and thereby gut granules, DHE staining was additionally observed in large and round storage compartments lacking GFP-RME-1 ( arrowheads). The sterol storage compartments in the glo-1 mutant were also different from rab7-positive late endosomes (not shown). These results extend our previous study by combining sterol bleach rate imaging with multicolour fluorescence microscopy. To unequivocally assess co-localization of DHE with the endosomal markers, we had to take into account a chromatic aberration (i.e., a focus shift between UV and green light in the microscope), as described previously for mammalian cells.Citation17 This allows us for the first time to identify sterol-containing organelles in a particular tissue of a whole animal. To identify the nature of the sterol-rich compartments in glo1-mutant animals, we performed additional co-localization studies: GFP-LMP1, a lysosomal marker, did not co-localize with DHE in these organelles (). The high sterol content of these organelles could be unequivocally verified from the corresponding bleach rate image calculated with PixBleach from the acquired bleach stacks (see above and ref. Citation7). Regions being rich in sterol showed the highest bleach rate constants, which can be used as a fingerprint to detect DHE.Citation7 GFP-RME8 is widely expressed endosomal marker in C. elegans, typically found on endosomes likely resembling sorting endosomes (SE) or multivesicular bodies (MVB) of mammalian cells.Citation18 Close inspection of double-labled adult glo-worms demonstrates that, at least some of the round sterol-containing organelles in the intestine of these animals have GFP-RME8 in their limiting membrane (). Similar observations were made with GFP-rab5, another marker for early endosomes (not shown). Together, these results show that in glo-mutant animals lacking intestinal gut granules fluorescent sterols become enriched in enlarged early endosomes being different from recycling endosomes and lysosomes.
Figures and Tables
Figure 1 Chemical structure of dehydroergosterol. Differences to ergosterol and cholesterol are indicated in red, while additional differences between DHE and cholesterol are indicated in green. Accordingly, DHE differs from ergosterol just in one additional double bound in the steroid backbone (red).
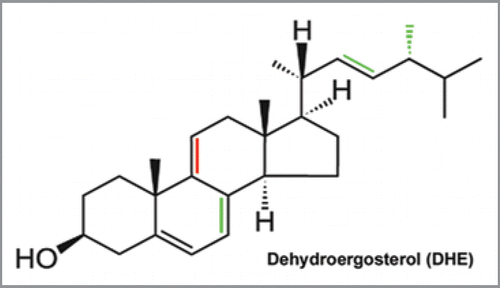
Figure 2 Bleach-rate-based image segmentation to visualize DHE in C. elegans. The figure illustrates the method of pixel-wise bleach-rate fitting used to distinguish probe fluorescence from autofluorescence in living nematodes. Various mathematical decay models, like a mono-exponential decay [mmo(t)] or stretched exponential decay [mst(t)] were fitted to the bleaching kinetics of the fluorescent sterol dehydroergosterol (DHE) in the nematode Caenorhabditis elegans in a pixel-wise manner. Images of DHE-stained worms were repeatedly acquired on a UV-sensitive wide field microscope to generate an input sequence. In every pixel position the intensity decay caused either by bleaching of DHE or of autofluorescence was fitted to the respective model, as exemplified for one pixel (white circle) in the diagram. Our software generates an amplitude map (upper green image) and a bleach rate map (lower image in FIRE LUT ).
![Figure 2 Bleach-rate-based image segmentation to visualize DHE in C. elegans. The figure illustrates the method of pixel-wise bleach-rate fitting used to distinguish probe fluorescence from autofluorescence in living nematodes. Various mathematical decay models, like a mono-exponential decay [mmo(t)] or stretched exponential decay [mst(t)] were fitted to the bleaching kinetics of the fluorescent sterol dehydroergosterol (DHE) in the nematode Caenorhabditis elegans in a pixel-wise manner. Images of DHE-stained worms were repeatedly acquired on a UV-sensitive wide field microscope to generate an input sequence. In every pixel position the intensity decay caused either by bleaching of DHE or of autofluorescence was fitted to the respective model, as exemplified for one pixel (white circle) in the diagram. Our software generates an amplitude map (upper green image) and a bleach rate map (lower image in FIRE LUT ).](/cms/asset/2941780d-d769-47bf-b086-6d76a856c116/kcib_a_10911972_f0002.gif)
Figure 3 Co-localization of DHE with GFP-tagged markers of the endosomal pathway. N2 nematodes (A) or glo1-mutant worms (B-D) expressing either GFP-RME 1 (A and B); GFP-RME 8 (C) or GFP-LMP1 (D) were labeled with DHE by feeding a DHE-cyclodextrin solution as described.Citation7 DHE was visualized by repeated imaging on a UV-sensitive wide field microscope and calculation of the bleaching kinetics of the sterol versus autofluorescence. The calculated amplitude image of the rapidly bleaching fraction resembled DHE and is shown in the most left panels of (C and D). Alternatively, in case of specimen movement, the DHE distribution was inferred from subtracting the last from the first frame of the stack (left in A and B), as described previously (ref. Citation7). Both methods gave identical results on sterol distribution. DHE was found in GFP-RME -1-positive basolateral recycling endosomes (arrows in A and B) and additional in large round storage organelles lacking this endosomal marker. These organelles also lacked GFP-LMP1 (arrowheads in D), but were surrounded by GFP-RME 8 (arrows in C, and inset). Sterol-containing tissue could be identified from the rapid bleaching of DHE being represented by a high bleach rate constant (exemplified for worms expressing GFP-LMP1; most right in D). High and low rate constants are given in blue/violet and orange/yellow, respectively. Bar, 20 µm.
Acknowledgements
D.W. acknowledges funding by the Danish Research agencies Forskningsstyrelsen, Forskningsrådet for Natur og Univers (FNU) and Forskningsrådet for Sundhed og Sygdom (FSS) and from thr Lundbeck foundation. Laboratory technician Tanja Christensen is acknowledged for help with nematode culture and experiments. Special thanks goes to Dr. Greg Herrmann (Dept. of Biology, Lewis and Clark College, Portland, OR, USA) who kindly provided the glo-mutant strains and the strains expressing GFP-tagged constructs.
Addendum to:
References
- Wüstner D. Intracellular cholesterol transport. In: Cellular lipid metabolism. Ed. Ehnholm C, Springer press 2009; 157 - 190
- Ji Y, Wang N, Ramakrishnan R, Sehayek E, Huszar D, Breslow JL, et al. Hepatic scavenger receptor BI promotes rapid clearance of high density lipoprotein free cholesterol and its transport into bile. J Biol Chem 1999; 274:33398 - 33402
- Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, et al. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature 2003; 421:268 - 272
- Entchev EV, Kurzchalia TV. Requirement of sterols in the life cycle of the nematode Caenorhabditis elegans. Semin Cell Dev Biol 2005; 16:175 - 182
- Matyash V, Geier G, Henske A, Mukherjee S, Hirsh D, Thiele C, et al. Distribution and transport of cholesterol in Caenorhabditis elegans. Mol Biol Cell 2001; 12:1725 - 1736
- Gerstbrein B, Stamatas G, Kollias N, Driscoll M. In vivo spectrofluorimetry reveals endogenous biomarkers that report healthspan and dietary restriction in Caenorhabditis elegans. Aging Cell 2005; 4:127 - 137
- Wüstner D, Landt Larsen A, Færgeman NJ, Brewer JR, Sage D. Selective visualization of fluorescent sterols in Caenorhabditis elegans by bleach-rate based image segmentation. Traffic 2010; 11:440 - 454
- Wüstner D, Brewer JR, Bagatolli LA, Sage D. Potential of ultraviolet widefield imaging and multiphoton microscopy for analysis of dehydroergosterol in cellular membranes. Microsc Res Tech 2010;
- Hermann GJ, Schroeder LK, Hieb CA, Kershner AM, Rabbitts BM, Fonarev P, et al. Genetic analysis of lysosomal trafficking in Caenorhabditis elegans. Mol Biol Cell 2005; 16:3273 - 3288
- Grant B, Hirsh D. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol Biol Cell 1999; 10:4311 - 4326
- Grant B, Zhang Y, Paupard MC, Lin SX, Hall DH, Hirsh D. Evidence that RME-1, a conserved C. elegans EH-domain protein, functions in endocytic recycling. Nat Cell Biol 2001; 3:573 - 579
- Lin SX, Grant B, Hirsh D, Maxfield FR. Rme-1 regulates the distribution and function of the endocytic recycling compartment in mammalian cells. Nat Cell Biol 2001; 3:567 - 572
- Hao M, Lin SX, Karylowski OJ, Wüstner D, McGraw TE, Maxfield FR. Vesicular and non-vesicular sterol transport in living cells. The endocytic recycling compartment is a major sterol storage organelle. J Biol Chem 2002; 277:609 - 617
- Gagescu R, Demaurex N, Parton RG, Hunziker W, Huber LA, Gruenberg J. The recycling endosome of Madin-Darby canine kidney cells is a mildly acidic compartment rich in raft components. Mol Biol Cell 2000; 11:2775 - 2791
- Wüstner D, Herrmann A, Hao M, Maxfield FR. Rapid nonvesicular transport of sterol between the plasma membrane domains of polarized hepatic cells. J Biol Chem 2002; 277:30325 - 30336
- Wüstner D. Improved visualization and quantitative analysis of fluorescent membrane sterol in polarized hepatic cells. J Microsc 2005; 220:47 - 64
- Wüstner D, Færgeman NJ. Chromatic aberration correction and deconvolution for UV sensitive imaging of fluorescent sterols in cytoplasmic lipid droplets. Cytometry A 2008; 73:727 - 744
- Zhang Y, Grant B, Hirsh D. RME-8, a conserved J-domain protein, is required for endocytosis in Caenorhabditis elegans. Mol Biol Cell 2001; 12:2011 - 2021