Abstract
Zebrafish belladonna (bel) mutants carry a mutation in the lhx2 gene that encodes a Lim domain homeobox transcription factor, leading to a defect in the retinotectal axon pathfinding. As a result, a large fraction of homozygous bel mutants is achiasmatic. Achiasmatic bel mutants display ocular motor instabilities, both reserved optokinetic response (OKR) and spontaneous eye oscillations, and an unstable swimming behavior, described as looping. All these unstable behaviors have been linked to the underlying optic nerve projection defect. Looping has been investigated under different visual stimuli and shown to be vision dependent and contrast sensitive. In addition, looping correlates perfectly with reversed OKR and the spontaneous oscillations of the eyes. Hence, it has been hypothesized that looping is a compensatory response to the perception of self-motion induced by the spontaneous eye oscillations. However, both ocular and postural instabilities could also be caused by a yet unidentified vestibular deficit. In this paper, we performed a preliminary test of the vestibular function in achiasmatic bel larval mutants in order to clarify the potential role of a vestibular deficit in looping. We found that the vestibular ocular reflex (VOR) is normally directed in both bel mutants and wild types and therefore exclude the possibility that nystagmus and looping in reverse to the rotating optokinetic drum can be attributed to an underlying vestibular deficit.
Recently, we suggested zebrafish belladonna (bel) mutant as an animal model for the study of human infantile nystagmus syndromeCitation1,Citation2 (INS, formerly called congenital nystagmus).Citation3 INS is a human ocular motor disorder that is characterized by involuntary conjugate, mostly horizontal uniplanar oscillations of both eyes, present at birth or shortly after.Citation4 Thus far, the etiology of INS is poorly understood.Citation5–Citation9 Through the typical accelerating slow phases and/or pendular eye motion, INS can be readily distinguished from other types of nystagmus.Citation5 Achiasmatic zebrafish bel mutants show an array of behaviors that closely mimic INS in humans. For instance, the waveforms of the ocular motor instabilities in zebrafish are strikingly similar to those in humans with INS.Citation1
Analogous to the compromised postural balance common to INS,Citation10–Citation12 zebrafish bel mutants show looping, a behavior that was linked to the ocular motor disorder in a recent publication of our laboratory.Citation13 In short, we hypothesized that looping may be a compensatory body movement driven by illusionary self-motion perception (circular vection), which in itself may be evoked by the ocular motor instabilities via exposure to constant high retinal slip velocity. This hypothesis was backed up by a number of experiments. Nystagmus always occurred prior to the start of looping when a stationary visual stimulus was provided. However, looping could also be triggered in the absence of eye movements (i.e., nystagmus) through ganzfeld motion (i.e., the moving scene generated the retinal slip necessary for circular vection). The direction of looping was determined by the ganzfeld motion but occurred in the opposite direction of the stimulus. Although strongly suggesting that looping is visually induced, these experiments fail to completely exclude the possible influence of a vestibular defect. Indeed, rotational behaviors are a common symptom of vestibular disorders in other animals.Citation14,Citation15
To address the question whether looping in achiasmatic zebrafish bel mutants is caused by a vestibular dysfunction, we first needed to overcome some technical difficulties: Unlike the optokinetic response (OKR), which is mature, robust and reliably measurable in zebrafish by 5 days post fertilization (dpf), the angular vestibular ocular reflex (aVOR; or rotational VOR, RVOR) was not detectable until 35 dpf in a previous study.Citation16 Looping, however, occurs as early as 5 dpf when the ocular motor instabilities (i.e., reversed OKR and nystagmus) are already manifest.Citation13 Thus, we had to develop a method for assessing the vestibular system at such an early stage.
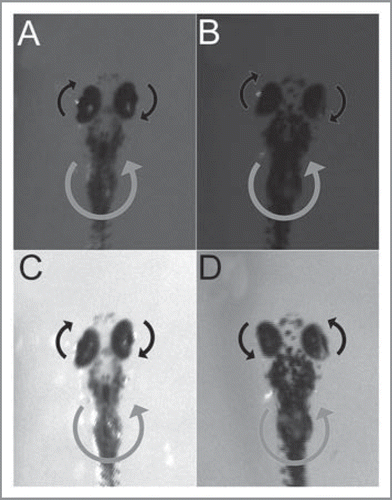
Larvae (5–6 dpf) were imbedded dorsal up in the center of a 35-mm-diameter Petri dish containing 3% methylcellulose in order to suppress whole-body motion without restricting eye movements. The dish was placed on a motorized horizontal turntable that was surrounded by a white uniform background. Body and eye movements were recorded by an infrared-sensitive CCD camera. Alternately rotating the larva on the turntable in dark with 2-s velocity steps failed to evoke a visible VOR, which is consistent with a previous study.Citation16 Yet, when we prolonged the angular velocity step to 10 s or more, we were able to detect eye movements opposite to the rotational direction of the turntable. The eyes then assumed a peripheral position, or sometimes drifted back to the center. The response was similar in both bel mutants and their heterozygous siblings (wild types) (; Movie S1). After light-on, we immediately observed an eye movement direction change in bel mutants, but not in wild types (; Movie S1). Although the environment was held at a minimum contrast, the area above the turntable may have provided enough contrast to trigger an OKR. Thus, the sudden change in eye movement direction in bel mutants may be accounted for by the reversed OKR that overrode the VOR. Additionally, the onset of the eye movement occurred earlier in both bel mutants and wild-type fish (VOR + OKR) when compared to the dark (pure VOR) (see Movie S1). The normally directed VOR in bel mutants excludes the possibility that nystagmus and looping in reverse to the rotating optokinetic drum can be attributed to an underlying vestibular defect.
Vestibular deficits that cause rotational behaviors in humans and animals are always asymmetric, and therefore associated with a unilateral vestibular problem.Citation14–Citation19 However, looping in individual zebrafish bel mutants lack directional preference. This is not surprising since all known morphological (gap between the lens and the retinal pigment epithelium, ipsilateral projection of the retinal ganglion cells)Citation20 and ocular motor phenotypes (reversed OKR and nystagmus)Citation1 are symmetrical, suggesting that the mutation in the zebrafish lhx2 homologCitation21 equally affects both sides. Hence, a vestibular dysfunction would most likely be bilateral, a case in which circular behavior is not expected. Furthermore, humans with bilateral vestibular deficits show impaired balance without vertigo.Citation22 Congenital or early-occurring vestibulopathy may go unnoticed and merely leads to a slightly unsteady gait in darkness.Citation17,Citation18,Citation22 Hence, the pronounced circling without directional preference in zebrafish bel mutants is neither consistent with a unilateral nor a bilateral vestibular problem.
Although the rotational VOR in zebrafish is not fully matured at the larval stage, it is present and can be assessed with prolonged velocity steps as described above. In addition to the use in zebrafish, this method may enable the measurement of the VOR in other small teleost and xenopus species at a much younger stage than previously thought possible.Citation16,Citation23,Citation24 Taking advantage of this novel experimental paradigm with prolonged velocity steps, it is now possible to investigate the development (or developmental pace) of the VOR in zebrafish. Additionally, this technique could be adapted to behavioral screens in order to identify genetically modified mutants with deficiencies in the vestibular system,Citation25,Citation26 or be applied as a test of earlystage VOR after genetic or pharmacological manipulations.Citation27–Citation30
Abbreviations
| bel | = | belladonna |
| INS | = | infantile nystagmus syndrome |
| OKR | = | optokinetic response |
| VOR | = | vestibular ocular reflex |
| dpf | = | days post fertilization |
Figures and Tables
Figure 1 Vestibular ocular reflex (VOr) in 6-dpf zebrafish larvae. In dark, wild-type siblings (A) and bel mutants (B) showed eye movements opposite to the angular direction of the turntable. under normal illumination, wild-type siblings continued to exhibit eye movements as in dark (C), whereas bel mutants displayed eye movements in the same direction as the turntable (d). Gray arrows depict the angular direction of the turntable, and black arrows depict the compensatory eye movement relative to the body.
Additional material
Download Zip (1.5 MB)Acknowledgements
This work was partly supported by the Swiss National Research Foundation (grant 3100A0-118069) and a cooperative project support by “Zurich Center for Integrative Human Physiology” (ZIHP) at the University of Zurich.
Addendum to:
References
- Huang Y-Y, Rinner O, Hedinger P, Liu S-C, Neuhauss SCF. Oculomotor instabilities in zebrafish mutant belladonna: a behavioral model for congenital nystagmus caused by axonal misrouting. J Neurosci 2006; 26:9873 - 9880
- Huang Y-Y, Neuhauss SCF. The optokinetic response in zebrafish and its applications. Front Biosci 2008; 13:1899 - 1916
- CEMAS Working Group. A national eye institute sponsored workshop and publication on the classification of eye movement abnormalities and strabismus (CEMAS) 2001; The National Eye Institute Publications
- Leigh RJ, Zee DS. The neurology of eye movements 2006; 4th edn. Oxford Oxford University Press
- Abadi RV. Mechanisms underlying nystagmus. J R Soc Med 2002; 95:231 - 234
- Optican LM, Zee DS. A hypothetical explanation of congenital nystagmus. Biol Cybern 1984; 50:119 - 134
- Broomhead DS, Clement RA, Muldoon MR, Whittle JP, Scallan C, Abadi RV. Modelling of congenital nystagmus waveforms produced by saccadic system abnormalities. Biol Cybern 2000; 82:391 - 399
- Jacobs JB, Dell’Osso LF. Congenital nystagmus: hypotheses for its genesis and complex waveforms within a behavioral ocular motor system model. J Vis 2004; 4:604 - 625
- Dell’Osso LF. Biologically relevant models of infantile nystagmus syndrome: the requirement for behavioral ocular motor system models. Semin Ophthalmol 2006; 21:71 - 77
- Guerraz M, Shallo-Hoffmann J, Yarrow K, Thilo KV, Bronstein AM, Gresty MA. Visual control of postural orientation and equilibrium in congenital nystagmus. Invest Ophthalmol Vis Sci 2000; 41:3798 - 3804
- Gresty M, Halmagyi GM. Head nodding associated with idiopathic childhood nystagmus. Ann N Y Acad Sci 1981; 374:614 - 618
- Eggert T, Straube A, Schroeder K. Visually induced motion perception and visual control of postural sway in congenital nystagmus. Behav Brain Res 1997; 88:161 - 168
- Huang Y-Y, Tschopp M, Neuhauss SCF. Illusionary self-motion perception in zebrafish. PLoS One 2009; 4:e6550
- Löscher W. Abnormal circling behavior in rat mutants and its relevance to model specific brain dysfunctions. Neurosci Biobehav Rev 2010; 34:31 - 49
- Rossmeisl JH. Vestibular disease in dogs and cats. Vet Clin North Am Small Anim Pract 2010; 40:81 - 100
- Beck JC, Gilland E, Tank DW, Baker R. Quantifying the ontogeny of optokinetic and vestibuloocular behaviors in zebrafish, medaka and goldfish. J Neurophysiol 2004; 92:3546 - 3561
- Casselbrant ML, Mandel EM. Balance disorders in children. Neurol Clin 2005; 23:807 - 829
- Kaga K. Vestibular compensation in infants and children with congenital and acquired vestibular loss in both ears. Int J Pediatr Otorhinolaryngol 1999; 49:215 - 224
- Hotson JR, Baloh RW. Acute vestibular syndrome. N Engl J Med 1998; 339:680 - 685
- Karlstrom RO, Trowe T, Klostermann S, Baier H, Brand M, Crawford AD, et al. Zebrafish mutations affecting retinotectal axon pathfinding. Development 1996; 123:427 - 438
- Seth A, Culverwell J, Walkowicz M, Toro S, Rick JM, Neuhauss SCF, et al. belladonna/(lhx2) is required for neural patterning and midline axon guidance in the zebrafish forebrain. Development 2006; 133:725 - 735
- Jen JC. Bilateral vestibulopathy: clinical, diagnostic and genetic considerations. Semin Neurol 2009; 29:528 - 533
- Lambert FM, Beck JC, Baker R, Straka H. Semicircular canal size determines the developmental onset of angular vestibuloocular reflexes in larval xenopus. J Neurosci 2008; 28:8086 - 8095
- Beck JC, Baker R. Static and dynamic measurement of otolithic vor during the absence of canal function in larval zebrafish. Soc Neurosci Abstr 2005; 31:391 - 316
- Brockerhoff SE, Hurley JB, Janssen-Bienhold U, Neuhauss SC, Driever W, Dowling JE. A behavioral screen for isolating zebrafish mutants with visual system defects. Proc Natl Acad Sci USA 1995; 92:10545 - 10549
- Haffter P, Granato M, Brand M, Mullins MC, Hammerschmidt M, Kane DA, et al. The identification of genes with unique and essential functions in the development of the zebrafish, danio rerio. Development 1996; 123:1 - 36
- Kawakami K. Transposon tools and methods in zebrafish. Dev Dyn 2005; 234:244 - 254
- Kotani T, Nagayoshi S, Urasaki A, Kawakami K. Transposon-mediated gene trapping in zebrafish. Methods 2006; 39:199 - 206
- Balciunas D, Wangensteen KJ, Wilber A, Bell J, Geurts A, Sivasubbu S, et al. Harnessing a high cargo-capacity transposon for genetic applications in vertebrates. PLoS Genet 2006; 2:169
- Ekker SC. Morphants: a new systematic vertebrate functional genomics approach. Yeast 2000; 17:302 - 306