Abstract
TRPC proteins have been implicated in a large array of Ca2+ signaling processes and are considered as pore-forming subunits of unique polymodal channel sensors. The mechanisms of TRPC activation are so far incompletely understood but appear to involve a concert of signals that are generated typically downstream of receptor-mediated activation of phospholipase C. Specifically for the TRPC1/4/5 subfamily the activating scenario is ill-defined and appears enigmatic due to the observation of multiple modes of activation. TRPC4 was initially described as a store-operated cation channel and was repeatedly proposed as a pivotal element of the store-operated signaling pathways of various tissues. However, classical reconstitution of TRPC4 complexes in expression systems as well as recent knock-down strategies provided evidence against store-dependent regulation of this channel and raised considerable doubt in its proposed prominent role agonist-induced Ca2+ signaling. Recent analysis of the function of TRPC4 in vascular endothelial cells of divergent phenotype revealed a novel aspect of TRPC signaling, extending the current concept of TRPC regulation by a phenotype-dependent switch between Ca2+ transport and a potential intracellular scaffold function of the TRPC protein.
The Enigmatic Gating Control of TRPC Channels
All seven members of the “classical” family of transient receptor potential (TRP) proteins are considered as pore forming subunits of non-selective cation channels, which are controlled by cellular phospholipase C (PLC) activity (reviewed in refs. Citation1–Citation4). Although the key role of PLC in channel activation is without controversy, the molecular mechanisms that govern TRPC channel gating downstream of PLC are in large part elusive. Historically, the TRPC subfamily received substantial interest immediately after identification of the mammalian trp genes, based on their potential function as sensors for depletion of intracellular Ca2+ stores,Citation5 and most members have been implicated in store-operated Ca2+ entry phenomena in various tissues.Citation6–Citation10 However, an initial, detailed analysis of the channel properties in heterologous expression systems strongly suggested that upon heterologous overexpression in classical host cells such as HEK293 or CHO, most TRPC species form receptor-regulated cation channels that are activated independently of the filling state of the endoplasmic reticulum (ER).Citation11–Citation14 For a subset of TRPC proteins (TRPC3/6 and 7), diacylglycerol was identified as an activating mediator that links these channels to phospholipase C activity.Citation11 Nonetheless, regulatory communication and even physical interaction with components of the ER has repeatedly been demonstrated for TRPC proteins including also TRPC3/6/7.Citation15–Citation22 Moreover, the potential importance of subunit heteromerization and the formation of signalcomplexes of certain stoichiometry was found to determine biophysical as well as regulatory properties of TRPC channels.Citation12,Citation23–Citation25 A particular tight relation between channel activity and the Ca2+ content of the ER appear to exist for the TRPC1/4/5 subset of closer relatives. The more recently identified ER Ca2+ sensor Stim1, which is a key component of store-depletion activated Ca2+ entry via Orai channels, has been demonstrated to interact also with TRPC1/4 and 5 proteins and may confer store-dependent regulation of certain TRPC channel multimers.Citation26–Citation28 Although several gating determinants, key factors and potential mechanisms of channel activation have been reported for TRPC1/4/5 channels, the molecular chain of events leading to activation is inclompletely understood. Processes potentially involved in TRPC4/5 channel activation include the targeting of channels into specific membrane domains and interaction with the actin cytoskeleton mediated by ERM-domain proteins,Citation29,Citation30 changes in local PIP2 content of the channels membrane environment and recruitment and activation of pertussis toxin sensitive G proteins.Citation31 Moreover local changes in free Ca2+ and/or divalent concentrations at the cytoplasic face of the channel complex are a likely determinant of channel gating.Citation32 Besides integration of lipid, Ca2+ and G-protein signals by the TRPC4 gating machinery, additional impact of physical coupling of TRPC4 channels to ER Ca2+ stores via electrostatic interaction with Stim1 needs consideration.Citation27 Similar to other TRP channels it appears feasible to speculate that multiple modes of TRPC4 activation might exist, depending on the composition, stoichiometry and cellular localization of the signalplex.
Plasticity of Signalplexes as the Basis of Polymodal TRPC Function
Variations in TRPC signaling complex composition and stoichiometry have been suspected to underly the divergent regulatory properties of TRPC channels observed along with variation of expression levels and cell types.Citation23,Citation24,Citation26,Citation33,Citation34 TRPC signalplex composition may change with expression and availablability of complex partners within a certain cellular background. We and others speculated that TRPC proteins may serve different cellular functions by recruitment into multiple distinct channel complexes.Citation35–Citation38 Consequently, the regulatory and (patho) physiological impact of a TRPC protein is expected to vary significantly with changes in the level of expression and cellular localization of signaling partners. This concept may explain differences in the function of a particular TRPC protein in divergent host cell environments. Nonetheless, gating modality of a TRPC protein are likely to change also within a given cell type along with changes cellular remodeling processes and phenotype switching.
TRPC4, a Contested Player in Vascular Endothelial (Patho)physiology
Evidence from a murine TRPC4 knock-down model suggested that this channel protein is of particular importance in the cardiovascular system, representing a prominent endothelial TRPC species.Citation9,Citation39 Consequently, the role of TRPC4 in endothelial physiology and pathophysiology has been analyzed extensively using a variety of approaches including siRNA as well as dominant negative knock-down strategies. These investigations yielded highly controversial results ranging from evidence for a key role of TRPC4 in the endothelial store-operated pathway, which controls essential functions such as barrier stability, gene expression and mediator production,Citation9,Citation39–Citation42 to a complete disqualification of TRPC4 as an endothelial Ca2+ transport system.Citation43 Interestingly, despite the latter report caused series doubt in a prominent Ca2+ signaling function of TRPC4 in the endothelium, the relevance of this protein as a determinant of endothelial proliferation was further confirmed.
A Phenotype Window Discloses TRPC4 Channel Function in Vascular Endothelium
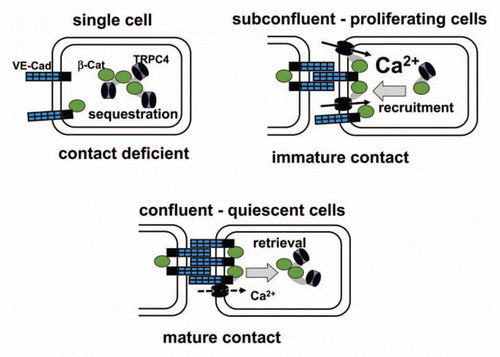
Recent mistrust in the involvement of TRPC4 in endothelial Ca2+ signaling was based on a convincing demonstration that store-operating Ca2+ entry into endothelial cells is mediated by the Stim1-Orai pathway.Citation43 Hence, the cellular role of vascular TRPCs became again misty with the glimpse of a possible ion transport independent function of these proteins. At the same time, we investigated agonist-induced changes in plasma membrane expression of endothelial TRPC4 proteins and observed a striking phenotype dependence of surface expression of the channel protein.Citation44 Most importantly, we failed to detect significant plasma membrane targeting of TRPC4 in single migrating endothelial cells. As this cellular state is typically the substrate for investigation of whole cell membrane conductances and also Ca2+ entry, our observation of unavailable TRPC4 membrane targeting was perfectly in line with the reported lack of Ca2+ signaling function.Citation43 Nonetheless, proliferating, sub-confluent populations displayed a marked recruitment of TRPC4 into the plasma membrane upon stimulation with either EGF or thrombin, and we identified a TRPC4-mediated Ca2+ signaling pathway specifically in proliferating clusters of endothelial cells that formed immature cell-cell contacts (). TRPC4 expression was without significant impact on Ca2+ signaling in single cells as well as in mature endothelial barriers. In conclusion, TRPC4 appears to function as a Ca2+ entry channel exclusively at a certain cellular state during endothelial development and/or barrier repair/regeneration. Close inspection of phenotype transition and detailed analysis of a rather small phenotype window enabled us to verify and characterize Ca2+ transport function of TRPC4 in native endothelial cells. The transient function of TRPC4 as part of a plasma membrane Ca2+ channel complex raises the question if TRPC4 and other TRPC proteins are able to adopt specific cellular functions when targeted to intracellular membranes and protein complexes. A respective hypothesis was put forward by our finding that TRPC4 associates with the junctional protein β-catenin.Citation44 This newly identified interaction partner represents another multifunctional signaling molecule that binds to TRPC4 also within the cell in nucleus associated compartments. Thus, TRPC4 may serve endothelial phenotype switching in a highly polymodal manner including intracellular crosstalk with other signaling pathways.
Perspectives and Outlook
The TRPC channel family is considered as a paradigm of multifunctional signaling molecules, based on their polymodal gating behavior and their typical promiscuity with respect to gating stimuli. The recently uncovered highly phenotype-dependent cellular localization and function of TRPC4 adds a new dimension to the concept of polymodal TRPC signaling. The observed intracellular targeting and integration into specific endothelial signalplexes including association and cotranslocation with β-catenin, opens the view on Ca2+ transport independent functions of TRPC proteins and crosstalk with important processes controlling gene expression such as the Wnt pathway. In aggregate, polymodal TRPC signaling appears to play a crucial role in phenotype transition within the vascular system. The potential role of TRPCs in tissue remodeling and repair needs particular attention and warrant further investigations.
Figures and Tables
Figure 1 Proposed model of phenotype-dependent TRPC4 function in growth factor-stimulated endothelial cells via interaction of the channel with β-catenin and targeting into cell-cell contacts. TRPC4 is for a large part sequestered in intracellular compartments and unavailable for Ca2+ signalling in single cells (contact deficient; upper left). By contrast, formation of immature cell adhesions promotes surface targeting of β-catenin-TRPC4 complexes and enables further recruitment of channels into the plasma membrane and Ca2+ entry function (immature contact; upper right). Once mature barriers are formed (mature contact; lower), TRPC4 resides for a large part in junctional complexes that are rapidly retrieved from the cell surface during growth factor stimulation and are barely available for contribution to global Ca2+ signaling.
Acknowledgements
I wish to thanks Dr. Michael Poteser for helpful discussions and FWF (P19820) as well as ÖNB (8216) for financial support.
References
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem 2007; 76:387 - 417
- Vazquez G, Wedel BJ, Aziz O, Trebak M, Putney JW Jr. The mammalian TRPC cation channels. Biochim Biophys Acta 2004; 1742:21 - 36
- Pedersen SF, Owsianik G, Nilius B. TRP channels: An overview. Cell Calcium 2005; 38:233 - 252
- Birnbaumer L. The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca(2+) concentrations. Annu Rev Pharmacol Toxicol 2009; 49:395 - 426
- Zhu X, et al. Trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell 1996; 85:661 - 671
- Liu X, et al. Trp1, a candidate protein for the store-operated Ca(2+) influx mechanism in salivary gland cells. J Biol Chem 2000; 275:3403 - 3411
- Ng LC, Gurney AM. Store-operated channels mediate Ca(2+) influx and contraction in rat pulmonary artery. Circ Res 2001; 89:923 - 929
- Groschner K, et al. Trp proteins form store-operated cation channels in human vascular endothelial cells. FEBS Lett 1998; 437:101 - 106
- Freichel M, et al. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nat Cell Biol 2001; 3:121 - 127
- Philipp S, et al. TRP4 (CCE1) protein is part of native calcium release-activated Ca2+-like channels in adrenal cells. J Biol Chem 2000; 275:23965 - 23972
- Hofmann T, et al. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 1999; 397:259 - 263
- Lintschinger B, et al. Coassembly of Trp1 and Trp3 proteins generates diacylglycerol- and Ca2+-sensitive cation channels. J Biol Chem 2000; 275:27799 - 27805
- Schaefer M, et al. Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J Biol Chem 2000; 275:17517 - 17526
- Zitt C, et al. Expression of TRPC3 in Chinese hamster ovary cells results in calcium-activated cation currents not related to store depletion. J Cell Biol 1997; 138:1333 - 1341
- Yuan JP, et al. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell 2003; 114:777 - 789
- Treves S, et al. Junctate is a key element in calcium entry induced by activation of InsP3 receptors and/or calcium store depletion. J Cell Biol 2004; 166:537 - 548
- Ma HT, et al. Requirement of the inositol trisphosphate receptor for activation of store-operated Ca2+ channels. Science 2000; 287:1647 - 1651
- Liao Y, et al. Functional interactions among Orai1, TRPCs and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci USA 2008; 105:2895 - 2900
- Kiselyov K, et al. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature 1998; 396:478 - 482
- Kiselyov K, Mignery GA, Zhu MX, Muallem S. The N-terminal domain of the IP3 receptor gates store-operated hTrp3 channels. Mol Cell 1999; 4:423 - 429
- Huang GN, et al. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol 2006; 8:1003 - 1010
- Woo JS, Kim do H, Allen PD, Lee EH. TRPC3-interacting triadic proteins in skeletal muscle. Biochem J 2008; 411:399 - 405
- Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 2001; 29:645 - 655
- Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem 2003; 278:39014 - 39019
- Poteser M, et al. TRPC3 and TRPC 4 associate to form a redox-sensitive cation channel-evidence for expression of native trpc3/trpc4 heteromeric channels in endothelial cells. J Biol Chem 2006; 281:13588 - 13595
- Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol 2007; 9:636 - 645
- Zeng W, et al. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol Cell 2008; 32:439 - 448
- Cheng KT, Liu X, Ong HL, Ambudkar IS. Functional requirement for Orai1 in store-operated TRPC1-STIM1 channels. J Biol Chem 2008; 283:12935 - 12940
- Mery L, Strauss B, Dufour JF, Krause KH, Hoth M. The PDZ-interacting domain of TRPC4 controls its localization and surface expression in HEK293 cells. J Cell Sci 2002; 115:3497 - 3508
- Obukhov AG, Nowycky MC. TRPC5 activation kinetics are modulated by the scaffolding protein ezrin/radixin/moesin-binding phosphoprotein-50 (EBP50). J Cell Physiol 2004; 201:227 - 235
- Otsuguro K, et al. Isoform-specific inhibition of TRPC4 channel by phosphatidylinositol 4,5-bisphosphate. J Biol Chem 2008; 283:10026 - 10036
- Blair NT, Kaczmarek JS, Clapham DE. Intracellular calcium strongly potentiates agonist-activated TRPC5 channels. J Gen Physiol 2009; 133:525 - 546
- Trebak M, Bird GS, McKay RR, Putney JW Jr. Comparison of human TRPC3 channels in receptor-activated and store-operated modes. Differential sensitivity to channel blockers suggests fundamental differences in channel composition. J Biol Chem 2002; 277:21617 - 21623
- Bergdahl A, et al. Plasticity of TRPC expression in arterial smooth muscle: correlation with store-operated Ca2+ entry. Am J Physiol Cell Physiol 2005; 288:872 - 880
- Trebak M, Vazquez G, Bird GS, Putney JW Jr. The TRPC3/6/7 subfamily of cation channels. Cell Calcium 2003; 33:451 - 461
- Groschner K, Rosker C. TRPC3: a versatile transducer molecule that serves integration and diversification of cellular signals. Naunyn Schmiedebergs Arch Pharmacol 2005; 371:251 - 256
- Liao Y, et al. A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry. Proc Natl Acad Sci USA 2009; 106:3202 - 3206
- Eder P, Groschner K. TRPC3/6/7: Topical aspects of biophysics and pathophysiology. Channels 2008; 2:94 - 99
- Freichel M, et al. Functional role of TRPC proteins in vivo: lessons from TRPC-deficient mouse models. Biochem Biophys Res Commun 2004; 322:1352 - 1358
- Cioffi DL, Stevens T. Regulation of endothelial cell barrier function by store-operated calcium entry. Microcirculation 2006; 13:709 - 723
- Tiruppathi C, et al. Impairment of store-operated Ca2+ entry in TRPC4(−/−) mice interferes with increase in lung microvascular permeability. Circ Res 2002; 91:70 - 76
- Tiruppathi C, Minshall RD, Paria BC, Vogel SM, Malik AB. Role of Ca2+ signaling in the regulation of endothelial permeability. Vascul Pharmacol 2002; 39:173 - 185
- Abdullaev IF, et al. Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ Res 2008; 103:1289 - 1299
- Graziani A, et al. Cell-cell contact formation governs Ca2+ signaling by TRPC4 in the vascular endothelium: Evidence for a regulatory TRPC4-{beta}-catenin interaction. J Biol Chem 2009; 285:4213 - 4223