Abstract
The physiological role of microRNAs (miRNAs) is widely appreciated as a fine-tuner to post-transcriptionally regulate the expression of multiple genes in the cells of origin. Here, we highlight two significant characteristics of miRNAs: (1) they are secreted from the producing cells, and (2) they can deliver the gene silencing signals between living cells in vitro and in vivo. The circulation of miRNAs in human body fluids can be provided with a logical explanation by our discovery that the release of miRNAs is actively controlled through a ceramide-dependent machinery associated with exosome secretion. This finding can contribute to the development of circulating miRNAs as diagnostic biomarkers for a variety of diseases. We also demonstrated that secretory miR-16 was transferred into prostate cancer PC-3M cells subcutaneously xenografted in nude mice, resulting in the suppression of its target gene. This result suggests that faithfully to their primary role, secretory miRNAs can function as a translational inhibitor in recipient cells as well. In conclusion, miRNAs are liberated from their incipient cells, whereby they can exert their full potentials as a silence master of gene expressions.
Growing evidence suggests that extracellular microRNAs (miRNAs) stably exist in human body fluids, including plasma, saliva and urine, although ribonucleases (RNases) also circulate throughout the body.Citation1 This finding indicates that miRNAs are excreted after they are contained in RNase-resistant lipid vesicles, such as exosomes and apoptotic bodies. However, very little is known about the secretory machinery of miRNAs.
Our recent study demonstrated that the secretion of miRNAs is triggered by the elevation of the cellular amount of ceramide, a bioactive sphingolipid, whose synthesis is tightly regulated by neutral sphingomyelinase 2 (nSMase2) ().Citation2 The amount of miRNAs in the culture media increased or decreased in the settings of overexpression or knockdown of nSMase2, respectively. We also revealed that treatment with a chemical compound, GW4869, which can inhibit the enzymatic activity of nSMase2, markedly blocked the secretion of miRNAs as well as exosomes. These data suggest that miRNAs are shed from their original cells via an exosome-dependent pathway that involves ceramide biosynthesis. The molecular dissection of miRNA secretory mechanisms is likely to assist in a better understanding why the expression of secretory miRNAs is perturbed during the development of various diseases, including cancer, diabetes and immune disorders.Citation3–Citation5 This finding may be beneficial to confer reliability and credibility to the diagnostic use of secretory miRNAs.
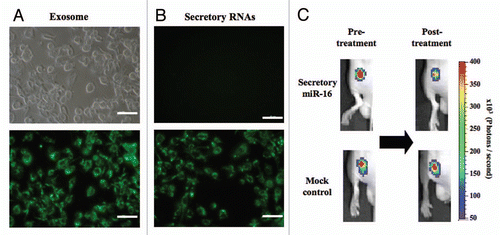
As evidenced by many reports,Citation6–Citation10 small RNAs are currently regarded as a category of intercellular signal entities, which are called, for instance, mobile small RNAs, systemic silencing signals or just secreted RNAs. To answer the question as to whether the secretory exosomal miRNAs that we observed can function biologically in a similar manner to other members, we set up in vitro and in vivo experiments pertaining to intercellular transfer. As shown in , purified exosomes labeled with a green lipophilic fluorescent reagent, PKH67, were successfully incorporated into recipient PC-3 cells. We also detected the migration of secretory nucleic acids into PC-3 cells by using SYTO dye, a specific probe for vital DNAs and RNAs including mRNAs and miRNAs (). Moreover, our latest publication reported that secretory miR-146a, a tumor-suppressive miRNA, lowered the expression of its target gene in the recipient cells, thereby inducing a phenotypic shift to cell growth inhibition. Taken together, the findings indicate that exosomal secretory miRNAs can spread translation-inhibitory signals, leading to the elicitation of a wide array of biological events.
If the movement of miRNAs is critical for living organisms, the transfer of silencing signals should be observed in some in vivo setting. We injected exosome fractions from a pri-miR-16 expression vector- or empty vector-transduced HEK293 cells into nude mice implanted with PC-3M-Fluc/Rluc-bcl2 3'UTR cells. This cell line was engineered to express renilla luciferase fused to the 3'UTR of bcl2, a validated miR-16 target gene, enabling us to monitor miR-16 activity.Citation11 As a result, the luciferase activity was suppressed by the intratumor injections of exosomes enriched in miR-16, whereas control exosomes did not alter the bioluminescence (). These data indicate that exosomal miR-16 delivered its inhibitory ability on its target gene to the recipient cells in our in vivo model. Taken together, the results of this study and those from the recent literaturesCitation12,Citation13 suggest that secretory miRNAs could play a pivotal role in the systemic signal network.
We propose the hypothesis that secretory miRNAs orchestrate a broad range of communication events, including intercellular to interindividual events. To our knowledge, the laboratory of Dr. Baulcombe was the first to identify mobile small RNAs as a silencing signal in plants.Citation14,Citation15 The discovery was astonishing because more than 10 years ago it was believed that extracellular nucleic acids were the result of cell death and lyses. Now, the spread of RNA interference between plant cells is recognized as a seminal genetic exchange mechanism implicated in virus resistance, genome maintenance and developmental control. Inasmuch as T cells are known to utilize exosomes for antigen presentation, miRNA delivery was anticipated between the immune cells.Citation16 As expected, Pegtel et al. revealed that viral miRNAs hijack the exosome secretory vesicles of host cells, causing the silencing of immunoregulatory genes in the recipient primary immature monocyte-derived DC cells.Citation9 This may compromise the local immune surveillance system, facilitating widespread infection in the whole body.
From a broad perspective, the relevance of miRNA transfer cannot be limited within an individual organism. We found that the expression of immune-related miRNAs in human breast milk culminates in the first 6 months of lactation, in agreement with the significance of colostrum in the context of passive immunity.Citation17 Importantly, this result appears to suggest the two following concepts: (1) dietary intake of miRNAs and (2) vertical transfer of miRNAs. If the miRNAs in food maintain their biological activity after digestion, they will be highly valued as a crucial ingredient that can modulate gene expression of our own cells. Tumor-suppressive miRNAs, in particular, could result in a commercial success in the healthy food business. Based on the second concept, genetic exchanges between mother and child would be mediated by exosomes contained in milk as well as amniotic fluids. Further analysis is needed to assess whether maternal miRNAs are delivered with functional integrity to infants. In addition to milk, the excretions from the body, such as tears, saliva, semen and vaginal discharge, also include secretory miRNAs,Citation3,Citation18–Citation20 suggesting that these fluids may act as a mediator of horizontal transfer. Secretory miRNAs might be a yet-to-be-discovered signal's identity of intriguing phenomena, for example, menstrual synchrony (the so-called dormitory effect).Citation21 It is within the bounds of probability that secretory miRNAs could be an interindividual communication tool among humans.
Here, we demonstrate that ceramide is a key stimulus for the secretion of miRNA-enriched exosomes and that secretory miRNAs can convey silencing signals to the recipient cells. It remains to be elucidated how miRNAs are loaded into exosomes and how secretory miRNAs are taken up by recipient cells. Although many questions are left open at this stage, we have been undergoing a paradigm shift to mobile miRNA-based signal exchange. Future studies may show the importance of secretory miRNAs in the intercellular and interindividual network.
Figures and Tables
Figure 1 A working model of secretory mechanism of microRNAs. Multivesicular bodies (MVBs) are an important cellular compartment for the metabolism of proteins and miRNAs. Ubiquitinated proteins are incorporated into lysosomes via endosomal sorting complex required for transport (ESCRT) machinery, subsequently followed by degradation or excretion. MiRNA s are packaged into exosomes and the release from the cells is stimulated by the surge of cellular ceramide.
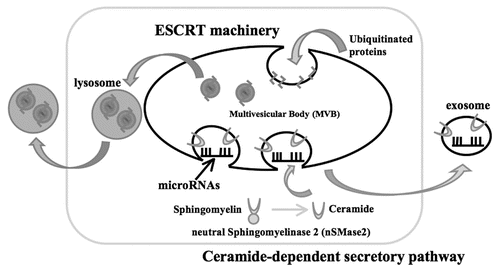
Figure 2 (A and B) Fluorescence photos of PC-3 cells incorporating PKH67-loaded exosomes (A) and SYTO13-labelled nucleic acids (B). (A) PC-3 cells were incubated for 24 h with PKH67-loaded exosomes (B) PC-3 cells were incubated for 24 h in the presence (lower photo) or absence (upper photo) of SYTO13-labelled conditioned medium. Emission at 514 nm was detected with Eclipse TE2000 Inverted Research Microscope and images were produced by NIS-Elements BR software. The size bar indicates 100 µm. (C) Transfer of exosomal miR-16 to PC-3M-Fluc/Rluc-bcl2 3'UTR cells in vivo. Six-week-old male Balb/c athymic nude mice were subcutaneously injected with 2 × 106 PC-3M cells into each dorsal region. One week after implantation, 0.2 mL of 14 µg protein/ml miR-16-enriched exosomes and control exosomes were injected into each tumor on day 0 and day 1. We carried out in vivo imagings on the pre- and 3-day-post-treatment with exosome fractions. For in vivo imaging, the mice were injected with ViviRen by intravenous tail vein injection and imaged immediately to count the photons from animal whole bodies using IVIS imaging system. Animal experiments in this study were performed in compliance with the guidelines of the Institute for Laboratory Animal Research, National Cancer Center Research Institute.
Acknowledgements
This paper was supported in part by a Grant-in-Aid for the Third-Term Comprehensive 10-Year Strategy for Cancer Control, a Grant-in-Aid for Scientific Research on Priority Areas Cancer from the Ministry of Education, Culture, Sports, Science and Technology and the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NiBio).
Addendum to:
References
- Fleischhacker M, Schmidt B. Circulating nucleic acids (CNAs) and cancer—a survey. Biochim Biophys Acta 2007; 1775:181 - 232
- Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 2010; 285:17442 - 17452
- Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 2008; 18:997 - 1006
- Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K, et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol 2008; 141:672 - 675
- Murata K, Yoshitomi H, Tanida S, Ishikawa M, Nishitani K, Ito H, et al. Plasma and synovial fluid microRNAs as potential biomarkers of rheumatoid arthritis and osteoarthritis. Arthritis Res Ther 2010; 12:86
- Dunoyer P, Schott G, Himber C, Meyer D, Takeda A, Carrington JC, et al. Small RNA duplexes function as mobile silencing signals between plant cells. Science 2010; 328:912 - 916
- Mlotshwa S, Voinnet O, Mette MF, Matzke M, Vaucheret H, Ding SW, et al. RNA silencing and the mobile silencing signal. Plant Cell 2002; 14:289 - 301
- Molnar A, Melnyk CW, Bassett A, Hardcastle TJ, Dunn R, Baulcombe DC. Small silencing RNAs in plants are mobile and direct epigenetic modification in recipient cells. Science 2010; 328:872 - 875
- Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci USA 2010; 107:6328 - 6333
- Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal 2009; 2:81
- Takeshita F, Patrawala L, Osaki M, Takahashi RU, Yamamoto Y, Kosaka N, et al. Systemic delivery of synthetic microRNA-16 inhibits the growth of metastatic prostate tumors via downregulation of multiple cell cycle genes. Mol Ther 2010; 18:181 - 187
- Rechavi O, Erlich Y, Amram H, Flomenblit L, Karginov FV, Goldstein I, et al. Cell contact-dependent acquisition of cellular and viral nonautonomously encoded small RNAs. Genes Dev 2009; 23:1971 - 1979
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9:654 - 659
- Baulcombe DC. RNA as a target and an initiator of post-transcriptional gene silencing in transgenic plants. Plant Mol Biol 1996; 32:79 - 88
- Voinnet O, Baulcombe DC. Systemic signalling in gene silencing. Nature 1997; 389:553
- Mignot G, Roux S, Thery C, Segura E, Zitvogel L. Prospects for exosomes in immunotherapy of cancer. J Cell Mol Med 2006; 10:376 - 388
- Kosaka N, Izumi H, Sekine K, Ochiya T. microRNA as a new immune-regulatory agent in breast milk. Silence 2010; 1:7
- Hanson EK, Lubenow H, Ballantyne J. Identification of forensically relevant body fluids using a panel of differentially expressed microRNAs. Anal Biochem 2009; 387:303 - 314
- Park NJ, Zhou H, Elashoff D, Henson BS, Kastratovic DA, Abemayor E, et al. Salivary microRNA: discovery, characterization and clinical utility for oral cancer detection. Clin Cancer Res 2009; 15:5473 - 5477
- Zubakov D, Boersma AW, Choi Y, van Kuijk PF, Wiemer EA, Kayser M. MicroRNA markers for forensic body fluid identification obtained from microarray screening and quantitative RT-PCR confirmation. Int J Legal Med 2010; 124:217 - 226
- McClintock MK. Menstrual synchrony and suppression. Nature 1971; 229:244 - 245