Abstract
Endocytosis is a fundamental process of membrane-trafficking in eukaryotes, but has not been known to occur in bacteria or archaea. The origin of endocytosis is central to the understanding of evolution of the first eukaryotes and their endomembrane systems. In a recent study we have established that an endocytosis-like process for uptake of proteins into cells occurs in a bacterium, Gemmata obscuriglobus, a member of the distinctive phylum Planctomycetes of peptidoglycan-less budding bacteria. Members of this phylum characteristically possess cells divided into compartments separated by internal membranes, and in the case of G. obscuriglobus these compartments include one where a double membrane envelope surrounds its nucleoid DNA, as well as an outer ribosome-free region of cytoplasm. Proteins can be internalized by cells from the external milieu and collected into this ribosome-free compartment, and this process is energy-dependent and appears to be receptor-mediated. As in eukaryote endocytosis, internalized proteins are associated with vesicles, and can be subjected to proteolytic degradation. The discovery of this process in a bacterium has significant implications for our understanding of the origins of endocytosis in eukaryotes.
Endocytosis is a cellular process for uptake of macromolecules from the external milieu by a mechanism involving plasma membrane infolding and vesicle formation. In the form of receptor-mediated endocytosis, it involves binding of the external macromolecular ligand to receptor molecules on the plasma membrane followed by trafficking of the receptor-ligand package to vesicles. The vesicles formed via plasma membrane infolding are called early endosomes; they are coated with a cage of clathrin and other proteins facilitating vesicle trafficking.Citation1–Citation3 Endocytosis seems to have been an ancient feature of eukaryotes, with evidence from phylogenetic analysis that its molecular machinery must have been present in the last eukaryote common ancestor (LECA).Citation4–Citation6 It is a process previously considered as eukaryote-specific, and was not known to occur in the domains Bacteria or Archaea, referred to together as prokaryotes. In bacterial cells import of amino acids and small peptides can occur but proteins have to be first degraded via extracellular proteases for them to be used as carbon, nitrogen and energy sources.
However, in a recent study we established that the planctomycete bacterium Gemmata obscuriglobus is able to uptake large folded proteins in a process somewhat similar to eukaryotic endocytosis.Citation7 Our conclusions were based on the following findings:
(a) Gemmata obscuriglobus cells can uptake full-length macromolecules in an energy-dependent manner. When cells were grown on agar medium and then incubated with proteins such as GFP, BSA, streptavidin, ovalbumin, horseradish peroxidase and immunoglobulin, they incorporated these proteins into the cell interior. This process is energy-dependent, since it is inhibited by sodium azide and non-permissive temperatures; ATP could restore the protein uptake after sodium azide inhibition.
(b) This protein uptake ability appears to be receptor-mediated, and the receptor appears relatively non-specific with respect to different proteins. DNA in the form of fluorescent oligonucleotides and plasmid DNA was not taken up, and did not compete with proteins for uptake, suggesting that there are limits to the relatively non-specific nature of the receptor with respect to proteins. Competition between different proteins can be inferred from experiments showing that if G. obscuriglobus cells were incubated with two different fluorescent proteins at the same concentrations, equivalent signals are seen inside cells. If one protein was in considerable excess, the signal for the other protein could not be detected inside cells. Quantitative experiments show inhibition of uptake of fluorescent protein in the presence of a non-fluorescent different protein. These experiments established that the proteins use only one receptor for internalization; a model in which protein added in excess occupies the receptor to the exclusion of the competing protein is consistent with the experimental results and such a mechanism.
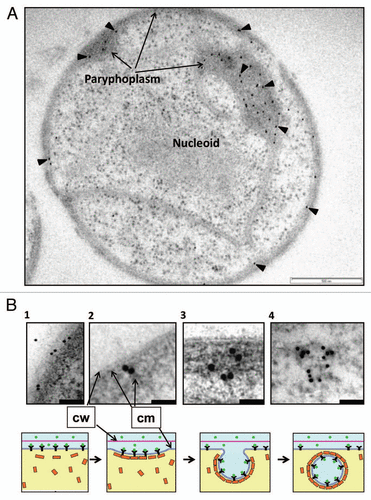
(c) Proteins taken up by G. obscuriglobus cells appear to localize to vesicles in a specific cell compartment in the outer region of the cell, the paryphoplasm. Immunogold labeling to localize GFP demonstrated this at EM level (). This ribosome-free compartment is one known from previous ultrastructural studies to be characteristic for planctomycete cells; it is defined on its outer side by the cytoplasmic membrane and cell wall, and on its inner side by a single intracytoplasmic membrane. Since it is part of the cell cytoplasm as defined by the cytoplasmic membrane boundary, it is not to be confused with a periplasm. Using differential centrifugation and fractionation experiments we found that internalized proteins are associated with membranes and vesicles, but not with the soluble fraction. If proteins were to enter the cell via a pore-driven mechanism, they would be expected to remain in the soluble fraction rather than with membranes. Such pores would have to be large indeed if such proteins as GFP or immunoglobulin were to be allowed to pass through the membrane in a folded state. The association of GFP with vesicles was also observed in the high-pressure frozen cryosubstituted cells (), although the paryphoplasm in Gemmata cells is quite dense so this causes problems for visualizing membranes within the vesicles. Gold particles indicating GFP are clearly associated with vesicle membrane rather than the interior of vesicles, which is entirely consistent with a mechanism in which protein ligand associates with cytoplasmic membrane receptors, and remains attached to those receptors as the vesicle infolds. A membrane-trafficking vesicle-mediated mechanism is relevant to ways in which Gemmata cells must transport materials internally, since the cell cytoplasm is compartmented into membrane-bounded regions—e.g., transcription must occur in the nuclear compartment and protein synthesis is unlikely to occur in the ribosome-free paryphoplasm, so proteins for the cell membrane and wall must be transported across the intracytoplasmic membrane at the least.
(d) We have found that an antibody raised against a G.obscuriglobus MC protein homologCitation8 reacts with vesicle-like structures within the paryphoplasm of G. obscuriglobus cells, and with vesicle membranes isolated by sub-cellular fractionation. Planctomycetes are known to be exceptional among Bacteria since they carry genes homologous to those coding for membrane coat (MC) proteins central to eukaryotic endocytosis.Citation8 MC proteins are related to the clathrin and COP families, all members of which are associated with vesicle formation or membrane curvature, and share common secondary structural features of an α solenoid combined with a β-propellor,Citation9 and some of which (e.g., clathrin) are necessary for receptor-mediated endocytosis.Citation1
(e) Internalized proteins are degraded in the paryphoplasm—this implies the existence of lysosome-like compartments with proteases, and trafficking of internalized proteins from endocytotic vesicles to those compartments. Conceivably such compartments contain other degradative enzymes—lysosomes possess aryl sulfatase among other degradative enzymesCitation10,Citation11 and it is of interest that G. obscuriglobus has a gene annotated as an arylsulfatase, consistent with the occurrence in the genome of another planctomycete, Rhodopirellula, of many annotated sulfatases.Citation12 This also suggests that some vesicles in the paryphoplasm should have acid pH internally, like lysosomes,Citation10 and also LAMP and other lysosomal membrane proteinsCitation13–Citation19 should be present; these predictions should be testable even though the small size of planctomycetes relative to eukaryotes makes this challenging.
From consideration of all these results, we proposed that Gemmata obscuriglobus has an ability to uptake proteins in a similar way to eukaryotic cells.Citation7 We deduce here a model based explaining how a simple receptor-mediated endocytosis may occur in G. obscuriglobus (). In the model, protein ligands bind to receptor molecules in the cytoplasmic membrane, and recruit clathrin-like membrane complex (MC) molecules which induce first a coated pit-like infolding of the cytoplasmic membrane followed by budding of a vesicle within the paryphoplasm. One of the resulting vesicles or vesicles derived later from these early vesicles may perform protein degradation.
We consider our finding as the first step in establishing the nature of endocytosis in bacteria. The next step will include the study of the molecular mechanism for this process. Although some data exist on occurrence of clathrin-like proteins in G. obscuriglobus,Citation8 homologs of other proteins such as adaptins, SNAREs, Rab GTPases etc., known to be necessary for endocytosis in eukaryotes should be found in this species. This is conceivable since Rab GTPases may be present in other bacteria.Citation20 Further experiments using pull-downs and investigation of existence of clathrin cages and triskelion formation from MC-like proteins should be undertaken. Another intriguing question is what kind of receptor for binding of proteins G. obscuriglobus possesses. This receptor is expected to be very unique as in contrast to the receptors of eukaryotes, it binds to a wide variety of proteins. Our work also raises a question of possible occurrence in these bacteria of an expected correlate of the endocytosis process, namely exocytosis where material is secreted from the cell via vesicle trafficking.
G. obscuriglobus is a member of the Planctomycetes, a phylum of budding and peptidoglycan-less bacteria sharing cell compartmentalization via internal membranes.Citation21,Citation22G. obscuriglobus is distinguished by its possession of a membranebounded envelope around its nucleoid consisting of two apposed membranes which is thus analogous to the nuclear envelope of eukaryote cells.Citation21–Citation23 The planctomycete phylum forms a so-called PVC superphylum with other bacterial phyla Verrucomicrobia and Chlamydiae among several other phyla.Citation24 Interestingly, verrucomicrobia also possess cells compartmentalized via internal membranes,Citation25 and also possesses MC proteins.Citation8 Clearly the endocytosis-like process we have found in one planctomycete species is relevant to our understanding of how eukaryote cell biology may have evolved. The steps to that understanding will involve deeper knowledge of mechanisms within the existing model organism G. obscuriglobus and also knowledge about the phylogenetic distribution of such mechanisms within related bacteria.
Figures and Tables
Figure 1 TEM of sectioned high-pressure frozen cryosubstituted G. obscuriglobus cell pre-incubated with GFP (A), and a proposed model of the events involved in uptake of extracellular proteins (B). The sectioned cell seen in (A) has been immunolabeled via anti-GFP antibody and the antibody visualized with 10 nm gold (arrowheads)—labeling is concentrated in the paryphoplasm region. Bar = 500 nm. (B) Micrographs in the top row show enlarged views of the potential different stages of GFP uptake at peripheral cytoplasmic membrane and paryphoplasm in cells of G. obscuriglobus. Bacteria were incubated with GFP, immunolabelled with anti-GFP antibody and processed and sectioned for TEM as for (A). Gold particles indicating GFP can be seen associated with cytoplasmic membrane (B1 and B2) and then with the membrane of vesicles inside the paryphoplasm region in the cell interior (B3 and B4). The schematic series of diagrams in the bottom row show corresponding suggested stages for the GFP uptake process and its mechanism, consistent with evidence from micrographs; stage 1 shows binding of GFP to membrane receptors, and stages 2 and 3 show initial steps of invagination of plasma membrane and association of GFP with vesicles in the process of being generated. At the final stage (stage 4) the vesicle formation is complete. In this model GFP ligand in the external milieu binds to receptors in the cytoplasmic membrane, MC-like (clathrin-like) protein coats the outside of the vesicle via cage formation and ligand becomes oriented to the inside membrane surface of the vesicle during its formation due to the effect of infolding. Infolding and formation of vesicles occurs in the paryphoplasm. Arrows indicate cytoplasmic membrane (cm) and cell wall (cw); green circles in the model indicate GFP; yellow rectangles, MC-like proteins; black Y's, protein receptor. Bars = 200 nm (micrograph B1), 50 nm (micrograph 2 and 3) and 100 nm (micrograph B4).
Addendum to:
References
- Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem 2009; 78:857 - 902
- Geli MI, Riezman H. Endocytic internalization in yeast and animal cells: similar and different. J Cell Sci 1998; 111:1031 - 1037
- Goldstein JL, Anderson RG, Brown MS. Coated pits, coated vesicles and receptor-mediated endocytosis. Nature 1979; 279:679 - 685
- Dacks JB, Field MC. Evolution of the eukaryotic membrane-trafficking system: origin, tempo and mode. J Cell Sci 2007; 120:2977 - 2985
- Field MC, Gabernet-Castello C, Dacks JB. Reconstructing the evolution of the endocytic system: insights from genomics and molecular cell biology. Adv Exp Med Biol 2007; 607:84 - 96
- Field MC, Dacks JB. First and last ancestors: reconstructing evolution of the endomembrane system with ESCRTs, vesicle coat proteins and nuclear pore complexes. Curr Opin Cell Biol 2009; 21:4 - 13
- Lonhienne TG, Sagulenko E, Webb RI, Lee KC, Franke J, Devos DP, et al. Endocytosis-like protein uptake in the bacterium Gemmata obscuriglobus. Proc Natl Acad Sci USA 2010; 107:12883 - 12888
- Santarella-Mellwig R, Franke J, Jaedicke A, Gorjanacz M, Bauer U, Budd A, et al. The compartmentalized bacteria of the Planctomycetes-Verrucomicrobia-Chlamydiae superphylum have membrane coat-like proteins. PLoS Biol 2009; 8:1000281
- Devos D, Dokudovskaya S, Alber F, Williams R, Chait BT, et al. Components of coated vesicles and nuclear pore complexes share a common molecular architecture. PLoS Biol 2004; 2:380
- Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem 1986; 55:663 - 700
- von Figura K, Hasilik A. Lysosomal enzymes and their receptors. Annu Rev Biochem 1986; 55:167 - 193
- Glöckner FO, Kube M, Bauer M, Teeling H, Lombardot T, Ludwig W, et al. Complete genome sequence of the marine planctomycete Pirellula sp. strain 1. Proc Natl Acad Sci USA 2003; 100:8298 - 8303
- Eskelinen EL, Tanaka Y, Saftig P. At the acidic edge: emerging functions for lysosomal membrane proteins. Trends Cell Biol 2003; 13:137 - 145
- Winchester BG. Lysosomal membrane proteins. Eur J Paediatr Neurol 2001; 5:11 - 19
- Braulke T, Bonifacino JS. Sorting of lysosomal proteins. Biochim Biophys Acta 2009; 1793:605 - 614
- Canuel M, Libin Y, Morales CR. The interactomics of sortilin: an ancient lysosomal receptor evolving new functions. Histol Histopathol 2009; 24:481 - 492
- Ni X, Canuel M, Morales CR. The sorting and trafficking of lysosomal proteins. Histol Histopathol 2006; 21:899 - 913
- Trowbridge IS. Endocytosis and signals for internalization. Curr Opin Cell Biol 1991; 3:634 - 641
- van Meel E, Klumperman J. Imaging and imagination: understanding the endo-lysosomal system. Histochem Cell Biol 2008; 129:253 - 266
- Yutin N, Wolf MY, Wolf YI, Koonin EV. The origins of phagocytosis and eukaryogenesis. Biol Direct 2009; 4:9
- Fuerst JA. Intracellular compartmentation in planctomycetes. Annu Rev Microbiol 2005; 59:299 - 328
- Lindsay MR, Webb RI, Strous M, Jetten MS, Butler MK, et al. Cell compartmentalisation in planctomycetes: novel types of structural organisation for the bacterial cell. Arch Microbiol 2001; 175:413 - 429
- Fuerst JA, Webb RI. Membrane-bounded nucleoid in the eubacterium Gemmata obscuriglobus. Proc Natl Acad Sci USA 1991; 88:8184 - 8188
- Wagner M, Horn M. The Planctomycetes, Verrucomicrobia, Chlamydiae and sister phyla comprise a superphylum with biotechnological and medical relevance. Curr Opin Biotechnol 2006; 17:241 - 249
- Lee KC, Webb RI, Fuerst JA. The cell cycle of the planctomycete Gemmata obscuriglobus with respect to cell compartmentalization. BMC Cell Biol 2009; 10:4