Abstract
Research suggests that social calls are important for conveying information about food and roost location in bats. However, no studies have specifically documented calls that are used to actively attract conspecifics to roosting locations. Here we describe the cooperative signaling behavior of roost location towards flying conspecifics in Spix’s disc-winged bat (Thyroptera tricolor), a species that uses a highly ephemeral roosting resource. Two types of calls were recorded during field experiments; one from flying individuals termed “inquiry calls”, and another from roosting bats termed “response calls”. Inquiry calls were emitted by flying bats immediately upon release, and quickly elicited production of response calls from roosting individuals. Most flying bats entered the roost when roosting individuals responded, while very few bats entered the roost in the absence of a response. During playback experiments, we found significant differences in response rates among individuals, which could be caused by diverse intrinsic and extrinsic factors. In addition, results of our ongoing field studies suggest that the cooperative signaling behavior of roost location is important in maintaining social cohesion, and that the use of a larger home range when resources are scarcer may decrease group stability by hindering communication.
Cooperation, a behavior performed by an individual that provides a benefit to a recipient, poses a problem to evolutionary theory because it potentially has negative fitness consequences on the performer. Thus, in the absence of a specific mechanism for the evolution of cooperation, natural selection should favor defectors. Notwithstanding, cooperative behaviors have been described in a wide diversity of taxa, including crustaceans, insects, fish, birds and mammals.Citation1 Types of cooperative behaviors include caring for young, grooming, sharing food, defense against predators, group hunting and sharing information about predators and food location.Citation2–Citation11 Information transfer about food location, in particular, has been a widely recognized cooperative behavior that has apparently played a major role in the evolution of sociality in birds and bats.Citation12,Citation13
Even though locating adequate food patches is a critical daily task for bats, finding and securing suitable roosting sites is also important, as roosts provide protection from predators and inclement weather, and also serve as sites for social and reproductive activities.Citation14 Bats use a wide diversity of structures for roosting, including caves, rock crevices, tree cavities and plants.Citation15 Depending on the ephemerality of a roost and the particular needs of the bats, such as avoiding predators and parasites, roosts may be used by individuals for several years or for very short periods of less than 24 hours.Citation16 Using such ephemeral roosts means that individuals need to locate suitable sites more often. Unfortunately, very little is known about how individuals locate sparse and ephemeral roosts. A few studies have suggested that acoustic signals may be an important cue used by bats to recruit conspecifics to roost-sites,Citation17–Citation19 but to date no research has examined if social calls are in fact actively used for this purpose.
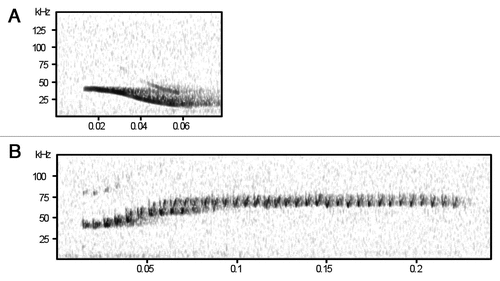
Our study of the social calls emitted by Spix's disk-winged bat (Thyroptera tricolor) provides, for the first time, conclusive evidence that a bat species uses cooperative signaling behaviors to convey information about roost location towards flying conspecifics.Citation20 Spix's disk wing bat is a neotropical species that maintains highly cohesive social groups,Citation21,Citation22 yet roosts in a very ephemeral habitat—the furled leaves of members of the order Zingiberales. In a series of field experiments, we found that when individuals are looking for roosts or roostmates, they emit “inquiry calls” () that often elicit a response from individuals who have already entered a furled leaf. These “response calls” () frequently drive the flying individual into the roost. Response calls appear to be emitted by roosting individuals intentionally to aid conspecifics in the location of roosts because they are emitted only after an audible inquiry call and vocalization of response calls ceases immediately after the flying individual enters the roost.
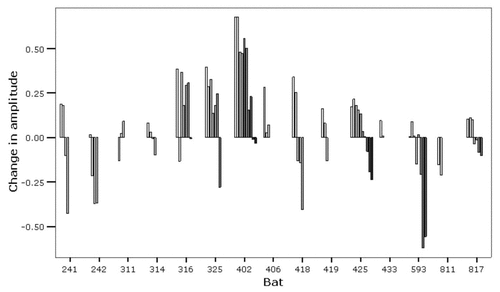
Results of acoustic trials show that flying bats typically emit an inquiry call immediately after being released, but do so rather infrequently (i.e., once every few seconds), while roosting bats emit numerous response calls in close succession. During these response bouts, the maximum amplitude of calls showed a decline with time (). Thus, response calls emitted right after the inquiry call were louder compared to response calls emitted later in a bout. We also observed that lactating females did not respond to inquiry calls or responded with only a single, weak call.
We conducted playback experiments with 53 bats, in which an individual was placed in a tubular leaf and presented with a series of recordings of inquiry calls. We found that response rates differ considerably between individuals, with 42% of bats responding quickly (i.e., immediately after the first inquiry call was broadcasted) and vigorously (i.e., loud and repetitive calls within a bout), to inquiry call playbacks. Another 19% responded, vigorously or not, within the first 10 broadcasted calls, while another 7% responded only after the same set of inquiry calls had been repeatedly broadcasted (i.e., after 20–30 calls). Thirtytwo percent of individuals sampled never responded to any of the inquiry calls broadcasted. Response rates did not appear to be influenced by factors such as time of day, temperature or time since capture, as individuals tested consecutively often differed significantly in their response to inquiry calls. These results suggest that there may be differences in cooperative rates among individuals, and that these rates may be influenced by factors such as reproductive status. Other factors that are known to affect cooperation include dispersal patterns, resource abundance, predation risk and group size,Citation23–Citation26 but whether any of these are relevant in explaining cooperation rates in T. tricolor remains to be tested.
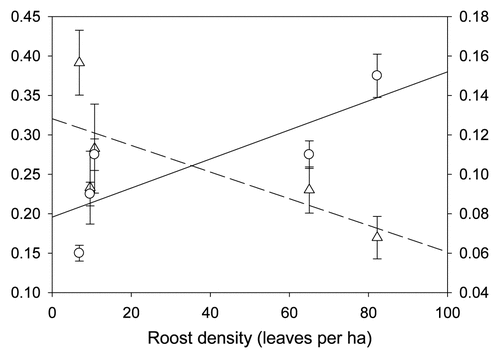
The cooperative signaling behavior of roost location towards flying conspecifics in T. tricolor may be an important means by which individuals help conspecifics reduce the costs associated with flight, such as high energetic expenditure and increased risk of predation,Citation27,Citation28 as these calls apparently increase the probability of finding roosts.Citation20 These social calls may also significantly increase the ability to locate group members, particularly if they contain specific signatures unique to each individual, increasing group stability. However, when bats inhabit areas of scarce roosting resources, individuals must travel long distances to locate suitable furled leaves and their social calls may not be audible to all group members due to atmospheric attenuation,Citation29 resulting in group fission. Our ongoing research on the social organization of T. tricolor shows that individuals use larger areas when roosts are scarcer and, as predicted, have lower association indices compared to individuals using smaller areas with abundant roosting resources (). Because a decrease in encounter rates is known to hinder reciprocation,Citation30 further research is necessary to understand if the cooperative signaling behavior of roost location observed in T. tricolor is more prevalent in populations that inhabit areas with a greater abundance of furled leaves.
Addendum to:
References
- Dugatkin LA. Cooperation among animals: an evolutionary perspective 1997; Oxford Oxford University Press
- Russell AF, Hatchwell BJ. Experimental evidence for kin-biased helping in a cooperatively breeding vertebrate. Proc R Soc Lond B 2001; 268:2169 - 2174
- Blumstein DT, Armitage KB. Cooperative breeding in marmots. Oikos 1999; 84:369 - 382
- Lehmann J, Korstjens AH, Dunbar RI. Group size, grooming and social cohesion in primates. Anim Behav 2007; 74:1617 - 1629
- Bulmer E, Celis P, Gil D. Parent-absent begging: evidence for sibling honesty and cooperation in the spotless starling (Sturnus unicolor). Behav Ecol 2008; 19:279 - 284
- Wilkinson GS. Reciprocal food sharing in the vampire bat. Nature 1984; 308:181 - 184
- Gilby IC, Eberly LE, Wrangham RW. Economic profitability of social predation among wild chimpanzees: individual variation promotes cooperation. Anim Behav 2008; 75:351 - 360
- Garay J. Cooperation in defence against a predator. J Theor Biol 2009; 257:45 - 51
- Wright J, Maklakov AA, Khazin V. State-dependent sentinels: an experimental study in the Arabian babbler. Proc R Soc Lond B 2001; 268:821 - 826
- Macdonald DW. The ecology of carnivore social behaviour. Nature 1983; 301:379 - 384
- Wilkinson GS. Information transfer at evening bat colonies. Anim Behav 1992; 44:501 - 518
- Ward P, Zahavi A. The importance of certain assemblages of birds as ‘information-centres’ for food finding. Ibis 1973; 115:517 - 534
- Safi K, Kerth G. Comparative analyses suggest that information transfer promoted sociality in male bats in the temperate zone. Am Nat 2007; 170:465 - 472
- Kunz TH. Kunz TH. Roosting ecology of bats. Ecology of Bats 1982; New York Plenum Press 1 - 50
- Kunz TH, Lumsden LF. Kunz TH, Fenton MB. Ecology of cavity and foliage roosting bats. Bat Ecology 2003; Chicago University of Chicago Press 3 - 87
- Lewis SE. Roost fidelity of bats: a review. J Mammal 1995; 76:481 - 496
- Kerth G, Reckardt K. Information transfer about roosts in female Bechstein's bats: an experimental field study. Proc R Soc Lond B 2003; 270:511 - 515
- Ruczynski I, Kalko EKV, Siemers BM. The sensory basis of roost finding in a forest bat, Nyctalus noctula. J Exp Biol 2007; 210:3607 - 3615
- Ruczynski I, Kalko EKV, Siemers BM. Calls in the forest: a comparative approach to how bats find tree cavities. Ethology 2009; 115:167 - 177
- Chaverri G, Gillam EH, Vonhof MJ. Social calls used by a leaf-roosting bat to signal location. Biol Lett 2010; 6:441 - 444
- Vonhof MJ, Whitehead H, Fenton MB. Analysis of Spix's disc-winged bat association patterns and roosting home ranges reveal a novel social structure among bats. Anim Behav 2004; 68:507 - 521
- Chaverri G. Comparative social network analysis in a leaf-roosting bat. Behav Ecol Sociobiol 2010; 64:1619 - 1630
- Krams I, Berzinš A, Krama T, Wheatcroft D, Igaune K, Rantala MJ. The increased risk of predation enhances cooperation. Proc R Soc Lond B 2010; 277:513 - 518
- Wagner K. Cooperative strategies and the evolution of communication. Artif Life 2000; 6:149 - 179
- Kümmerli R, Gardner A, West SA, Griffin AS. Limited dispersal, budding dispersal and cooperation: an experimental study. Evolution 2009; 63:939 - 949
- Ohtsuki H, Hauert C, Lieberman E, Nowak MA. A simple rule for the evolution of cooperation on graphs and social networks. Nature 2006; 441:502 - 505
- Thomas SP, Suthers RA. The physiology and energetics of bat flight. J Exp Biol 1972; 57:317 - 335
- Fenton MB, Rautenbach IL, Smith SE, Swanepoel CM, Grosell J, van Jaarsveld J. Raptors and bats: threats and opportunities. Anim Behav 1994; 48:9 - 18
- Bradbury JW, Vehrencamp SL. Principles of animal communication 1998; Sunderland, MA Sinauer Associates
- Ferriere R, Michod RE. The evolution of cooperation in spatially heterogeneous populations. Am Nat 1996; 147:692 - 717