Abstract
Synapse remodeling, which involves changes in the synaptic structure and their molecular composition, is required for the maturation and refinement of neural circuits. Although synapse remodeling is known to be tightly dependent on the assembly of local actin cytoskeleton, how actin directs the structural changes of synapse and targeting of synaptic proteins are not fully understood. Recently, we identified ephexin1, a Rho guanine nucleotide exchange factor (GEF) that regulates actin dynamics, plays an essential role in the maturation and functioning of the mammalian neuromuscular junction (NMJ). We showed that ephexin1 regulates the synaptic organization of the neurotransmitter receptor acetylcholine receptor (AChR) clusters through RhoA-dependent actin reorganization. Interestingly, ephexin1 has been implicated in the regulation of postsynaptic structure as well as the presynaptic vesicle release at various types of synapses. Our findings thus establish a novel function of ephexin1 in synapse remodeling through regulating the synaptic targeting of neurotransmitter receptors, revealing a versatile role of ephexin1 at synapses.
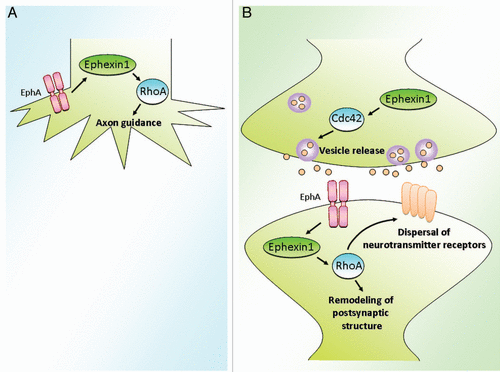
The precise function of neural circuits is determined by accurate synaptic connections between neurons and their target cells. The dynamic regulation of the formation, maturation and elimination of individual synapses represents a key process during the development and refinement of the neural circuits.Citation1 Such synapse remodeling is believed to be tightly controlled by the actin cytoskeleton, a fundamental element for directing the shaping of the synaptic architecture as well as the accumulation of synaptic proteins, especially neurotransmitter receptors, to the synaptic sites.Citation2 A number of actin regulators, such as the Rho GTPase family (including RhoA, Rac1 and Cdc42), have been shown to be crucial for synapse remodeling.Citation3 Work from our laboratory recently reported that ephexin1, a Rho guanine nucleotide exchange factor (GEF), controls the postsynaptic maturation of the mammalian neuromuscular junction (NMJ), a specialized synapse between the motor neuron and skeletal muscle. We revealed that ephexin1 functions to destabilize the neurotransmitter receptor acetylcholine receptors (AChRs) through weakening the cytoskeletal anchorage of the receptors in a RhoA-dependent manner. Importantly, this effect of ephexin1 is essential for the proper synaptic transmission at adult NMJ and may thus be important for certain motor responses such as the maintenance of muscle strength. Our finding reveals a pivotal role of ephexin1 in synapse remodeling by regulating the synaptic organization of neurotransmitter receptors ().
We demonstrated that the ephexin1-dependent RhoA activation leads to the dissociation of AChR clusters from the actin cytoskeleton, followed by disassembly of these receptor clusters. Importantly, ROCK-mediated pathway is required for this ephexin1-dependent receptor cluster dispersal. RhoA-ROCK-mediated pathways have been implicated in membrane trafficking and endocytic pathways,Citation4 and one of its downstream effectors cofilin has been reported to regulate the synaptic organization of AChR clusters through actin-dependent vesicular trafficking.Citation5 It is therefore possible that synaptic AChR receptors are removed by ephexin1 via cofilin-mediated endocytosis, a hypothesis that awaits further investigation. Notably, Rho GTPase-dependent pathways have also been shown to regulate the synaptic targeting of other types of neurotransmitter receptors in the brain, such as AMPA and NMDA receptors at the glutamatergic excitatory synapses of the central nervous system (CNS). Similar to AChRs, AMPA and NMDA receptors are associated with the actin cytoskeleton through specific scaffolds and actin-binding proteins.Citation6,Citation7 In addition, regulation of the actin dynamics has been shown to modulate the localization, recruitment and trafficking of both AMPA and NMDA receptors.Citation8,Citation9 For example, Oligophrenin1, a Rho GTPase-activating protein (GAP) whose mutations are associated with mental retardation, is involved in regulating the stability of AMPA receptors through downregulation of RhoA.Citation10,Citation11 In particular, activation of Oligophrenin1 upon synaptic activity inactivates RhoA, which leads to the decrease of AMPA receptor endocytosis and enhancement of synaptic transmission. Given that ephexin1 is abundantly expressed in the brain,Citation12 it will be of interest to investigate whether ephexin1 is a critical Rho GEF for the synaptic targeting of AMPA receptors through regulating RhoA activity in CNS neurons.
Synaptic strength is controlled by both the remodeling of neurotransmitter receptors and the re-organization of synaptic structure. We showed that in addition to regulating the maturation of AChR clusters, ephexin1 is also important for remodeling the postsynaptic structure at the NMJ. Ephexin1 regulates the proper formation of junctional folds, specialized postsynaptic membrane invaginations at the NMJ that increase the postsynaptic area as well as spatially separate different membrane receptors.Citation13 A number of proteins that link AChR receptors to the extracellular matrix and intracellular cytoskeleton, including the dystrophinglycoprotein complex (DGC) and neural cell-adhesion molecule (NCAM), are essential for junctional fold formation.Citation14–Citation16 How ephexin1, either through RhoAdependent mechanism or cooperate with the DGC or NCAM, controls the formation of junctional folds remains to be elucidated. Ephexin1 also plays an important role in regulating postsynaptic structure in CNS neurons. Our previous finding revealed that ephexin1 is involved in the retraction of dendritic spines, which are the specialized dendritic protrusions where most excitatory synapses in the brain are located.Citation17 The structural remodeling of dendritic spines mainly depends on the dynamics of local actin cytoskeleton.Citation2,Citation3 Ephexin1 was shown to be a critical molecule that mediates ephrin-Eph signaling towards activation of RhoA, leading to local actin rearrangement and retraction of the spines.Citation17 This effect of ephexin1 is important for proper synaptic transmission. Indeed, RhoA have been well characterized for its role in dendritic spine maintenance and synapse remodeling.Citation3,Citation18 Furthermore, dysregulation of RhoA has been reported in neurological disorders including mental retardation.Citation19 It will thus be pivotal to elucidate whether ephexin1 is a critical target for the altered RhoA activity during pathological conditions.
Besides being an essential Rho GEF in the postsynaptic apparatus, the role of ephexin1 for presynaptic function is beginning to be unraveled. Ephexin1 was initially identified to be important for the guidance of growing axons.Citation12,Citation20 The fact that ephexin1 is enriched in the axonal growth cones makes ephexin1 a potential regulator of presynaptic development, when growth cones develop into presynaptic specializations following synapse formation. Recent study on Drosophila NMJ indicates that presynaptic ephexin, the Drosophila homolog of ephexin1, plays a crucial role in the homeostatic modulation of vesicle release.Citation21 Surprisingly, this effect of ephexin does not require RhoA, but instead depends on Cdc42, another member of the Rho GTPase family. The detailed mechanism underlying how ephexin1 regulates vesicle release remains to be determined. In this context, it will be important to investigate whether ephexin1 exerts similar presynaptic function at the mammalian glutamatergic synapses.
Figures and Tables
Figure 1 Multiple roles of the Rho GEF ephexin1 during synapse formation and remodeling. (A) During early development of synapse formation, ephexin1 is an important regulator for the axon guidance of retina ganglion cells via activation of RhoA.Citation12,Citation20 (B) At mature hippocampal synapses and the neuromuscular junction, ephexin1 plays important roles at both pre- and postsynaptic sites.Citation17,Citation21 Presynaptically, ephexin1 regulates the homeostatic vesicle release, as shown at the drosophila NMJ. Postsynaptically, ephexin1 modulates both the localization of neurotransmitter receptors and the synaptic structural remodeling. In particular, ephexin1 functions to disperse the synaptic AChR clusters at the mammalian NMJ. In addition, ephexin1 is important for the structural remodeling of both the postsynaptic muscle membrane of the NMJ and the dendritic spines of the hippocampal neurons.
Acknowledgements
We thank Dr. Kwok-On Lai for helpful discussions and critical reading of the manuscript. This study was supported in part by the Research Grants Council of Hong Kong (6421/05M, 661007 and HKUST 1/06C), the Area of Excellence Scheme of the University Grants Committee (AoE/B15/01) and the Hong Kong Jockey Club.
Addendum to:
References
- Cohen-Cory S. The developing synapse: construction and modulation of synaptic structures and circuits. Science 2002; 298:770 - 776
- Dillon C, Goda Y. The actin cytoskeleton: integrating form and function at the synapse. Annu Rev Neurosci 2005; 28:25 - 55
- Tada T, Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol 2006; 16:95 - 101
- Qualmann B, Mellor H. Regulation of endocytic traffic by Rho GTPases. Biochem J 2003; 371:233 - 241
- Lee CW, Han J, Bamburg JR, Han L, Lynn R, Zheng JQ. Regulation of acetylcholine receptor clustering by ADF/cofilin-directed vesicular trafficking. Nat Neurosci 2009; 12:848 - 856
- Bolton MM, Blanpied TA, Ehlers MD. Localization and stabilization of ionotropic glutamate receptors at synapses. Cell Mol Life Sci 2000; 57:1517 - 1525
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 2002; 25:103 - 126
- Allison DW, Gelfand VI, Spector I, Craig AM. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. J Neurosci 1998; 18:2423 - 2436
- Zhou Q, Xiao M, Nicoll RA. Contribution of cytoskeleton to the internalization of AMPA receptors. Proc Natl Acad Sci USA 2001; 98:1261 - 1266
- Khelfaoui M, Pavlowsky A, Powell AD, Valnegri P, Cheong KW, Blandin Y, et al. Inhibition of RhoA pathway rescues the endocytosis defects in Oligophrenin1 mouse model of mental retardation. Hum Mol Genet 2009; 18:2575 - 2583
- Nadif Kasri N, Nakano-Kobayashi A, Malinow R, Li B, Van Aelst L. The Rho-linked mental retardation protein oligophrenin-1 controls synapse maturation and plasticity by stabilizing AMPA receptors. Genes Dev 2009; 23:1289 - 1302
- Shamah SM, Lin MZ, Goldberg JL, Estrach S, Sahin M, Hu L, et al. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell 2001; 105:233 - 244
- Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci 2001; 2:791 - 805
- Grady RM, Zhou H, Cunningham JM, Henry MD, Campbell KP, Sanes JR. Maturation and maintenance of the neuromuscular synapse: genetic evidence for roles of the dystrophin—glycoprotein complex. Neuron 2000; 25:279 - 293
- Rafuse VF, Polo-Parada L, Landmesser LT. Structural and functional alterations of neuromuscular junctions in NCAM-deficient mice. J Neurosci 2000; 20:6529 - 6539
- Adams ME, Kramarcy N, Fukuda T, Engel AG, Sealock R, Froehner SC. Structural abnormalities at neuromuscular synapses lacking multiple syntrophin isoforms. J Neurosci 2004; 24:10302 - 10309
- Fu WY, Chen Y, Sahin M, Zhao XS, Shi L, Bikoff JB, et al. Cdk5 regulates EphA4-mediated dendritic spine retraction through an ephexin1-dependent mechanism. Nat Neurosci 2007; 10:67 - 76
- Saneyoshi T, Fortin DA, Soderling TR. Regulation of spine and synapse formation by activity-dependent intracellular signaling pathways. Curr Opin Neurobiol 2010; 20:108 - 115
- Boda B, Dubos A, Muller D. Signaling mechanisms regulating synapse formation and function in mental retardation. Curr Opin Neurobiol 2010; In press
- Sahin M, Greer PL, Lin MZ, Poucher H, Eberhart J, Schmidt S, et al. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron 2005; 46:191 - 204
- Frank CA, Pielage J, Davis GW. A presynaptic homeostatic signaling system composed of the Eph receptor, ephexin, Cdc42 and CaV2.1 calcium channels. Neuron 2009; 61:556 - 569