Abstract
In Drosophila, the elimination of viable but suboptimal cells is mediated by cell competition, ensuring that these cells do not accumulate during development. In addition, certain genes such as the Drosophila homologue of human c-myc (dmyc) are able to transform cells into supercompetitors, which eliminate neighboring wild-type cells by apoptosis and overproliferate leaving total cell numbers unchanged. We have recently identified Drosophila SPARC as an early marker transcriptionally upregulated in loser cells that provides a transient protection by inhibiting caspase activation in outcompeted cells. Here, we explore whether the expression of SPARC in human tumors is consistent with a role for cell competition during human cancer and find that, consistent with the existence of competitive interactions between cancer and normal cells, SPARC is upregulated at the tumor-host boundaries in several types of human cancer.
During their lifetime, cells of multicellular animals can suffer insults (mutations, transient errors, infections, oxidative damage) that may compromise their fitness.Citation1 In Drosophila there are mechanisms (i.e., “cell competition”) that seem to recognize and eliminate less adapted or weaker cells of developmental primordia, ensuring that viable but suboptimal cells do not accumulate during development or ageing.Citation1 How groups of cells compare their relative fitness levels and decide which cell will remain in the tissue (winner cell) and which cell will die (loser cell) is becoming increasingly clear. Recent work in the Drosophila imaginal discs has shown that cell competition is a multistep process.
First, an insult (i.e., a mutationCitation2,Citation3), increases or decreases the fitness of a particular cell within the imaginal disc epithelium. Second, different isoforms of the cell membrane protein Flower (Fwe) label cells as winners or losers.Citation4 In Drosophila, the fwe locus produces three isoforms: Fweubi, FweLose-A and FweLose-B that function as a code.Citation4 Basal levels of Fweubi are constantly produced in the wing imaginal disc. During cell competition the loser cells stop producing Fweubi and the two FweLose isoforms are expressed instead. The name Flower was borrowed from the so called “Flower Wars” that was how the Aztecs called the battles fought in Central America between them and their neighbors, before the arrival of the Europeans.Citation5,Citation6 This was a peculiar type of ancient war where the Aztec warriors were trained to prefer capturing their enemies in battle rather than killing them. Therefore, losers were not killed immediately, but captured, then marked as “losers” with blue paint and eventually sacrificed later during an independent ritual. Our results in Drosophila suggested that the gene flower worked similarly during “cellular wars.” It is required for distinguishing loser cells from winner cells after competition for resources.Citation4 The different isoforms of Flower tag cells as Losers (expressing the Lose-A and/or Lose-B isoforms) or winners (cells expressing the ubi isoform) but the eventual death of the loser cells depends on other signals. Therefore, like the Aztecs, the defeated enemies are first identified as losers by marking their bodies and eventually killed in an independent ritual.Citation4–Citation6 Finally, this process results in a novel type of proliferation, where the winner cells proliferate to replace the loser cells.Citation7 This type of proliferation requires the killing of the surrounding cells and has been termed “apoptosis dependent-proliferation”.Citation1
It is interesting to note that certain genes, especially the Drosophila homologue of the proto-oncogene c-myc, dmyc, are able to transform cells into supercompetitors,Citation1 which are cells that are able to eliminate neighboring wild-type (wt) cells by apoptosis. Because the expansion of the supercompetitor cells by “apoptosis-dependent proliferation” occur at the expense of normal surrounding cells, total cell numbers do not change and morphological malformations are difficult to detect.Citation1,Citation7 Therefore it has been proposed that supercompetition and apoptosis-dependent proliferation could play an important role in early stages of cancer and the formation of “cancerization fields,” that occur in the absence of morphological malformations.Citation1,Citation8
SPARC in Cell Competition
We have recently identified dSparc, the Drosophila homologue of the Sparc/Osteonectin protein family as an early marker that is transcriptionally upregulated in loser cells during cell competition, acting as a self-protective signal for loser cells.Citation9 This mechanism allows useful cells to recover from transient and limited stress before they are unnecessarily eliminated by their neighbors.
Here, we explore whether the role of SPARC during cell competition could be conserved in humans, and in particular during human tumor formation. Our conclusion is that the role of SPARC during cell competition helps to understand the complex behavior of human and mouse Sparc at tumor-host borders.
dSparc, Tumorigenesis and Tumor Progression
Humans have three different dSparc homologs: SPARC/Osteonectin, SPARC like protein 1 and Follistatin-like protein among which SPARC/Osteonectin is best studied.Citation10 SPARC has been clearly implicated in tumor development, but published reports suggest diverse and contradictory functions: SPARC shows differential expression in many epithelial malignancies (prostate, lung, intestine),Citation10 and can promote metastasis.Citation11
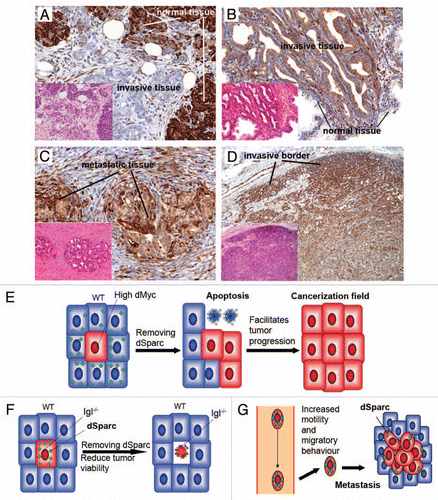
In order to evaluate possible similarities between competing cells and human cancers, we have stained a series of human tumor samples with anti-human SPARC antibodies to look for differential SPARC expression at the borders of neoplastic lesions and surrounding stroma (). Interestingly, higher SPARC expression levels were detected in normal tissue when compared to tumor areas in adenocarcinomas associated with field cancerization like Barrett's esophagus, ductal adenocarcinoma and mucinous cystic neoplasia of the pancreasCitation12 ( and data not shown).
On the contrary, higher SPARC levels were detected in tumor areas of adenoma and adenocarcinoma of the colon, urothelial carcinoma and prostatic acinar adenocarcinoma, when compared to surrounding normal tissueCitation13,Citation14 ( and data not shown). We also found increased expression in metastatic tissues and in the stroma surrounding urothelial carcinoma, invasive ductal carcinoma of the breast, prostatic acinar adenocarcinoma, adenocarcinoma associated with Barrett's esophagus, adenocarcinoma of the colon and pancreatic ductal adenocarcinoma, suggesting that SPARC may also be a useful marker to detect cell competition between cells of different histotype ().
Finally, we observed increased expression of SPARC in tumor cells located at borders of meningiomas and urothelial carcinoma, suggesting that these marginal cells behave differently from the bulk of the tumor, and may be engaged in competitive interactions at those edges ().
Our data obtained from the study of dSparc in cell competition support a model that might explain tumor-specific differences of SPARC function in human cancers. A subset of tumors may behave as “supercompetitors”Citation1,Citation7 (e.g., tumors that contain c-myc duplications) and trigger activation of SPARC in the neighboring stromal cells.Citation9 In this case, loss of SPARC will accelerate the elimination of normal tissue by the cancerous (supercompetitor) cells (). Other types of tumors may be recognized by the same system as suboptimal (i.e., tumor cells mutant for human lgl homologs),Citation1 SPARC upregulation will be triggered accordingly,Citation9 and a removal of SPARC will therefore reduce their viability (). Likewise, SPARC may promote metastasis,Citation10,Citation11 not only because it increases motility and migratory behavior, but also because SPARC upregulation may allow cancer cells to survive competitive interactionsCitation9 once they seed into soil tissues ().
Materials and Methods
Immunohistochemical stainings for SPARC/Osteonectin on human tissues.
Immunohistochemical staining for Sparc/Osteonectin was performed on formalin-fixed paraffin-embedded tissue blocks with an automated diagnostic system (Ventana Medical Systems, Inc.). Briefly, 3 µm thick sections were obtained with a microtome, transferred onto adhesive slides and dried at 62°C for 30 min. Immunohistochemistry was performed with mouse anti-human osteonectin monoclonal antibody (AON-5031, Hematologic Technologies), followed by biotinylated anti-mouse antibody and peroxidase-labeled streptavidin using a labeled streptavidin-biotin kit with 3,3′-diaminobenzidine chromogen as substrate. A positive control with a meningioma sample was present on each slide. Slides were counterstained with hematoxylin.
Figures and Tables
Figure 1 Similarities in the role of Sparc between cell competition and human cancers. (A–D), Serial tissue sections were stained with H&E (small parts) and with immunohistochemistry for SPARC (brown). (A) represents pancreatic ductal adenocarcinoma showing decreased SPARC compared with the surrounding normal parenchima of the pancreas. (B) the opposite pattern of SPARC expression is evident: prostatic acinar adenocarcinoma is characterized by increased expression of SPARC and the surrounding normal glands have barely detectable levels. (C) Metastasis, to spermatic cord, of prostatic acinar adenocarcinoma shows even higher levels of SPARC. (D) cells located at the invasive borders of meningiomas are characterized by strong expression of SPARC, when compared to the remaining tumor cells. Original magnification for (A–C), ×200; for (D), ×100. (E) In a first type of tumors, supercompetitors expand by killing normal surrounding cells that in the absence of SPARC cannot defend themselves. This type of tumors may form cancerization fields. (F) A second type of tumors (lethal-giant-larvae like) may have fitness problems and activate SPARC that allows them to survive. (G) Metastatic cells may survive better in soil tissues protecting themselves from a foreign environment upregulating SPARC.
Acknowledgements
We thank Marta Portela for help with . This work is funded by the European Research Council, Caja Madrid, Mutua Madrileña Science Foundation, Swiss National Science Foundation and the autonomous community of Madrid. The authors declare no competing interests.
Addendum to:
References
- Moreno E. Is cell competition relevant to cancer?. Nat Rev Cancer 2008; 8:141 - 147
- Morata G, Ripoll P. Minutes: mutants of drosophila autonomously affecting cell division rate. Dev Biol 1975; 42:211 - 221
- Moreno E, Basler K, Morata G. Cells compete for decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature 2002; 416:755 - 759
- Rhiner C, Lopez-Gay JM, Soldini D, Casas-Tintó S, Martín FA, Moreno E. Flower forms an extracellular code that reveals the fitness of a cell to its neighbors in Drosophila. Dev Cell 2010; 18:882 - 883
- Moreno E. A war-prone tribe migrated out of Africa to populate the world. Nature 2010; Available at http://hdl.handle.net/10101/npre.2010.4303.1
- Hassig R. Aztec Warfare: Imperial Expansion and Political Control. Civilization of the American Indian series, no. 188 1988; Norman, OK University of Oklahoma
- Moreno E, Basler K. dMyc transforms cells into super-competitors. Cell 2004; 117:117 - 129
- Rhiner C, Moreno E. Super competition as a possible mechanism to pioneer precancerous fields. Carcinogenesis 2009; 30:723 - 728
- Portela M, Casas-Tinto S, Rhiner C, López-Gay JM, Domínguez O, Soldini D, et al. Drosophila SPARC is a self-protective signal expressed by loser cells during cell competition. Dev Cell 2010; 19:562 - 573
- Clark CJ, Sage EH. A prototypic matricellular protein in the tumor microenvironment—where there's SPARC, there's fire. J Cell Biochem 2008; 104:721 - 732
- Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature 2005; 436:518 - 524
- Mantoni TS, Schendel RR, Rodel F, Niedobitek G, Al-Assar O, Masamune A, et al. Stromal SPARC expression and patient survival after chemoradiation for non-resectable pancreatic adenocarcinoma. Cancer Biol Ther 2008; 7:1806 - 1815
- Best CJ, Gillespie JW, Yi Y, Chandramouli GV, Perlmutter MA, Gathright Y, et al. olecular alterations in primary prostate cancer after androgen ablation therapy. Clin Cancer Res 2005; 11:6823 - 6834
- Lapointe J, Li C, Higgins JP, van de Rijn M, Bair E, Montgomery K, et al. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci USA 2004; 101:811 - 816