Abstract
Following T-cell antigen receptor (TCR) engagement, a multi-molecular complex consisting of SLP-76, Nck, and VAV1 is formed and recruited to the T-cell antigen-presenting-cell (APC) interaction site. This complex is crucial for the regulation of the actin machinery. The molecules Nck (an adaptor) and VAV1 (a GEF for small G-proteins) were previously shown to bind SLP-76. Using high-resolution imaging techniques, together with gene silencing and biochemical analysis, we studied the dynamics of this signaling complex formation. We recently showed that VAV1 and Nck can bind each other independently of SLP-76. This direct interaction is mediated by the binding of the Nck C-terminal SH3 domain and the VAV1 N-terminal SH3 domain. This interaction contributes to the cooperative nature of the complex formation. This observation was confirmed in functional studies: disruption of the Nck-VAV1 interaction strongly inhibited actin polymerization. Here, we show that Nck-VAV1 interaction is not required for Ca2+ mobilization, since a point mutation in the VAV1 N-terminal SH3 domain, which prevent the direct interaction between Nck and VAV1, has no effect on Ca2+ flux and minimal effects on ZAP-70, LAT, or PLCγ1 phosphorylation.
Following T-cell antigen receptor (TCR) engagement, multiprotein signaling complexes are formed at the interface of the T-cell and antigen-presenting-cell (APC). These complexes are required for biochemical signal transduction events that regulate numerous basic T-cell functions including cell proliferation, adhesion and motility, all crucial for the T-cell-mediated immune response.Citation1–Citation3 One fundamental cellular process regulating T-cell functions is the reorganization of actin-cytoskeleton and accumulation of actin filaments at the site of the T-cell/APC interface.Citation4–Citation6 A triple molecular complex consisting of the adapter molecules SLP-76, Nck and VAV1, regulates the Wiskott Aldrich Syndrome protein (WASp)—one of the key regulators of actin machinery in hematopoietic cells.Citation7 Nck and VAV1 play a role in the recruitment of WASp to the T-cell/APC contact site and in its activation, respectively. Previously, we and others have shown that the recruitment of WASp to the TCR site is enabled by SLP-76, which serves as a scaffold protein and is associated with Nck and VAV1. In our recently published study, we discovered that the Nck C-terminal SH3 domain binds the VAV1 N-terminal SH3 domain directly, and independently of SLP-76.Citation8 We showed that the binding of VAV1 to Nck occurs via the same site through which VAV1 binds to the Grb2 C-terminal SH3 domain.Citation9,Citation10
Sequential alignment between the SH3 C-terminal domains of Nck and Grb-2 showed high homology between the two.Citation9,Citation10 Since Grb-2 is a binding protein of VAV1, this suggests that Nck may mimic or even compete with Grb-2 on its binding domain. The possible competition between Nck and Grb-2 is supported by previous publications attributing the potential function of Nck to its structural similarity to Grb-2.Citation9,Citation10
Grb-2 associates with Sos, which functions as GEF of Ras proteins.Citation11 Previously, Sos was described as a potential partner of Nck.Citation12,Citation13 In vivo experiments have shown that exogenous expression of Nck results in a direct association with Sos.Citation13,Citation14 Since Sos functions as a GEF for the Rho proteins, it is possible that Nck also acts as a Grb-2 competitor for VAV1 binding site, similarly to the competition occurring for Sos binding.
We showed using in vivo experiments that this binding is important for actin polymerization.Citation8 Nck binds WASp via Nck's C-terminal SH3 domain, which was demonstrated in this study as a VAV1 binding site. This suggests that the nucleation promoting factor (NPF) which participates at the alternative actin polymerization pathway we proposed, is apparently not WASp. Furthermore, no T-cell development or signaling occurs in the absence of LAT or SLP-76. These molecules are required for the formation of critical signaling complexes. Indeed, we and others have studied the central region of SLP-76, which contributes to the highly cooperative interaction of LAT, Gads and PLCγ1.Citation15 By binding this region, Grb2 also can mediate the oligomerization of signaling complexes, a process which contributes to signaling at low receptor occupancy.Citation16 We also showed that dynamics of WASp-dependent actin pathways is impaired in SLP-76 deficient T cells.Citation5 In the absence of SLP-76, WASp remains at the cytosol and is not recruited to the T-cell membrane.Citation5 These data strengthen the above hypothesis suggesting a possible mechanism for actin polymerization in the absence of SLP-76, induced by an alternative NPF, other than WASp.
To investigate whether VAV1-Nck interaction influences proximal T-cell signaling cascades that regulate T-cell functions, we introduced a point mutation at the VAV1-binding site to Nck (W637A), and compared the phosphorylation profile of proximal signaling molecules of VAV1-deficient T cells (J-VAV) reconstituted with VAV1 W637A to that of cells reconstituted with VAV1 wt.
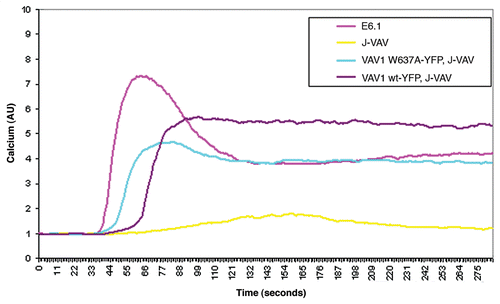
The phosphotyrosine profile of J-VAV lysate reconstituted with the VAV1 W637A mutant was found to be qualitatively similar to cells reconstituted with VAV1 wt (). Our data clearly indicated that this mutation of the VAV1 binding site (W637A) to Nck did not alter the phosphorylation of ZAP-70, LAT or PLCγ. These results were supported by the ability of J-VAV stably transfected with wt or the mutant W637A to exhibit normal Ca2+ flux () and NFAT transcriptional activity.Citation8 Calcium flux of J-VAV reconstituted with wt protein was similar to W637A and dramatically different from that of the non-reconstituted J-VAV, as previously published in reference Citation17. Our data clearly suggest that Nck-VAV1 interaction specifically regulates actin polymerization.
In the recently published study, we further explored the interaction of VAV1 and Nck with the N-terminal region of SLP-76.Citation8 VAV1 and Nck were known to interact with this SLP-76 domain, but their binding affinity and specificity were recently further characterized.Citation8 VAV1 and Nck may interact more efficiently in the presence of SLP-76, yet their direct binding suggests an additional potential cooperative interaction at SLP-76. The role of this interaction in actin polymerization indicates the significance of these two proteins for T-cell activity, and demonstrates for the first time, an additional role of Nck and VAV1 in T-cell signaling that is independent of SLP-76. It seems that the complexity of these interactions enables the formation of a large variety of complexes, with distinct functions. Such heterogeneity might allow for various forms of regulation and control, which in turn may affect T-cell function at various developmental stages or in various effector states.
Figures and Tables
Figure 1 Analysis of VAV1 deficient cells (J-VAV) and J-VAV stably expressing VAV1 wt or VAV1 W637A for their tyrosine phosphorylation profile. Unstimulated (−) and stimulated (+) cells were lysed and whole cell lysates were resolved on SDS-PAGE and probed with anti-pTy antibody to compare tyrosine phosphorylation profiles.
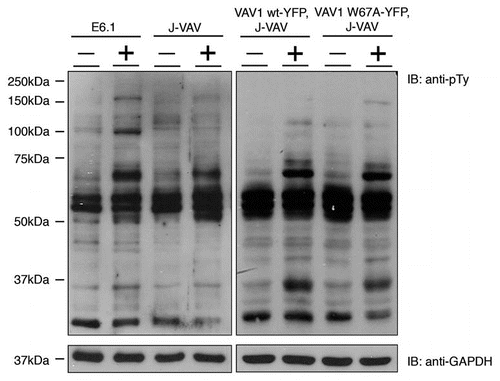
Figure 2 E6.1, J-VAV and stably transfected J-VAV expressing VAV1 wt or VAV1 W637A were loaded with Fura red-3. The cells were incubated with 5 µM Fluo-3 and 0.5 mM probenecid in RPMI 1640 medium at 37°C for 45 min. The cells were washed and maintained at room temperature for 30–45 min. Then, the cells were incubated at 37°C for 5 min and analyzed for Ca+2 flux. Baseline Ca+2 level was measured for 30 s, after which time OKT3 (150 ng/ml) was added. Ca+2 flux was measured for 4 min. Two independent experiments were performed.
Acknowledgements
This work was supported by the Israel Science Foundation (grants no. 971/08 and 1659/08).
Addendum to:
References
- Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 1998; 92:83 - 92
- Raab M, da Silva AJ, Findell PR, Rudd CE. Regulation of Vav-SLP-76 binding by ZAP-70 and its relevance to TCR zeta/CD3 induction of interleukin-2. Immunity 1997; 6:155 - 164
- Houtman JC, Brown PH, Bowden B, Yamaguchi H, Appella E, Samelson LE, et al. Studying multisite binary and ternary protein interactions by global analysis of isothermal titration calorimetry data in SEDPHAT: application to adaptor protein complexes in cell signaling. Protein Sci 2007; 16:30 - 42
- Zeng R, Cannon JL, Abraham RT, Way M, Billadeau DD, Bubeck-Wardenberg J, et al. SLP-76 coordinates Nck-dependent Wiskott-Aldrich syndrome protein recruitment with Vav-1/Cdc42-dependent Wiskott-Aldrich syndrome protein activation at the T cell-APC contact site. J Immunol 2003; 171:1360 - 1368
- Barda-Saad M, Braiman A, Titerence R, Bunnell SC, Barr VA, Samelson LE. Dynamic molecular interactions linking the T cell antigen receptor to the actin cytoskeleton. Nat Immunol 2005; 6:80 - 89
- Nolz JC, Gomez TS, Zhu P, Li S, Medeiros RB, Shimizu Y, et al. The WAVE2 complex regulates actin cytoskeletal reorganization and CRAC-mediated calcium entry during T cell activation. Curr Biol 2006; 16:24 - 34
- Bubeck Wardenburg J, Pappu R, Bu JY, Mayer B, Chernoff J, Straus D, et al. Regulation of PAK activation and the T cell cytoskeleton by the linker protein SLP-76. Immunity 1998; 9:607 - 616
- Barda-Saad M, Shirasu N, Pauker MH, Hassan N, Perl O, Balbo A, et al. Cooperative interactions at the SLP-76 complex are critical for actin polymerization. EMBO J 2010; 29:2315 - 2328
- Ye ZS, Baltimore D. Binding of Vav to Grb2 through dimerization of Src homology 3 domains. Proc Natl Acad Sci USA 1994; 91:12629 - 12633
- Nishida M, Nagata K, Hachimori Y, Horiuchi M, Ogura K, Mandiyan V, et al. Novel recognition mode between Vav and Grb2 SH3 domains. EMBO J 2001; 20:2995 - 3007
- Egan SE, Giddings BW, Brooks MW, Buday L, Sizeland AM, Weinberg RA. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature 1993; 363:45 - 51
- Hu Q, Milfay D, Williams LT. Binding of NCK to SOS and activation of Ras-dependent gene expression. Mol Cell Biol 1995; 15:1169 - 1174
- McCarty JH. The Nck SH2/SH3 adaptor protein: a regulator of multiple intracellular signal transduction events. BioEssays 1998; 20:913 - 921
- Buday L, Wunderlich L, Tamas P. The Nck family of adapter proteins: regulators of actin cytoskeleton. Cell Signal 2002; 14:723 - 731
- Houtman JC, Higashimoto Y, Dimasi N, Cho S, Yamaguchi H, Bowden B, et al. Binding specificity of multiprotein signaling complexes is determined by both cooperative interactions and affinity preferences. Biochemistry 2004; 43:4170 - 4178
- Houtman JC, Yamaguchi H, Barda-Saad M, Braiman A, Bowden B, Appella E, et al. Oligomerization of signaling complexes by the multipoint binding of GRB2 to both LAT and SOS1. Nat Struct Mol Biol 2006; 13:798 - 805
- Reynolds LF, Smyth LA, Norton T, Freshney N, Downward J, Kioussis D, et al. Vav1 transduces T cell receptor signals to the activation of phospholipase C-gamma1 via phosphoinositide-3-kinase-dependent and -independent pathways. J Exp Med 2002; 195:1103 - 1114