Abstract
Recently we reported that Dictyostelium cells ingest Legionella pneumophila by macropinocytosis, whereas other bacteria, such as Escherichia coli, Mycobacterium avium, Neisseria meningitidis or Salmonella typhimurium, are taken up by phagocytosis.1 In contrast to phagocytosis, macropinocytosis is partially inhibited by PI3K or PTEN inactivation, whereas both processes are sensitive to PLC inhibition. Independently from reduced uptake, L. pneumophila proliferates more efficiently in PI3K-null than in wild type cells. PI3K inactivation also neutralizes resistance to infection conferred by constitutively expressing the endo-lysosomal iron transporter Nramp1. We have shown this to be due to altered recruitment of the V-H+ ATPase, but not Nramp1, in the Legionella-containing vacuole (LCV) early during infection.1 As further evidence for impaired LCV acidification we examine here the effects of disrupting the small G protein RacH on Legionella infection.
Phagocytosis and macropinocytosis can be discriminated in Dictyostelium by several criteria, including requirement for tight particle binding for phagocytosis under shaking, differential involvement of membrane phosphoinositides in both processes, absence of macropinocytosis in wild type natural isolates. By exploiting these features, we have provided evidence that Legionella is taken up by macropinocytosis.Citation1 In particular we have shown both that Legionella uptake is negligible in wild type natural isolates, which are strictly dependent on phagocytosis for bacterial ingestion, and that uptake by axenic strains, which are capable of macropinocytosis, is strongly reduced under shaking but stimulated upon sodium meta-periodate pre-treatment of the bacteria, favoring the idea that Dictyostelium cells do not possess membrane receptors for Legionella. Finally, we showed that Legionella uptake is reduced upon genetic or pharmacological inactivation of PI3K or the antagonistic PTEN phosphatase.Citation1 In Dictyostelium as well as macrophages, PI3K inactivation partially inhibits fluid-phase macropinocytosis as well as phagocytosis of large particles, such as yeast, whereas fails to inhibit phagocytosis of ∼1 µm bacteria, such as E. coli.Citation1–Citation8
As additional evidence in favor of macropinocytosis, we have now tested a RacH-null mutant, that was generated and characterized by Somesh et al.Citation9 RacH belong to the Rho family of small GTPases that are ubiquitously distributed across the eukaryotes. By interacting with various effectors, these GTPases regulate the actin cytoskeleton and other cellular responses.Citation9,Citation10 In Dictyostelium, the Rho family includes 18 members mostly belonging to the Rac subfamily.Citation11 Some members of this family have been shown to regulate cell shape, spontaneous and chemotactic motility and phagocytosis.Citation11 RacH gene disruption resulted in inhibition of macropinocytosis, whereas phagocytosis as well as cell motility and chemotaxis were unaffected.Citation9 We found that Legionella uptake was strongly reduced in the mutantCitation1 (), thus supporting the notion that Legionella is taken up by macropinocytosis. We further investigated whether RacH inactivation affects intracellular proliferation of L. pneumophila and found that Legionella multiplied better in RacH-null cells than in the parental AX2 cells (), thus resembling PI3K-null cells.
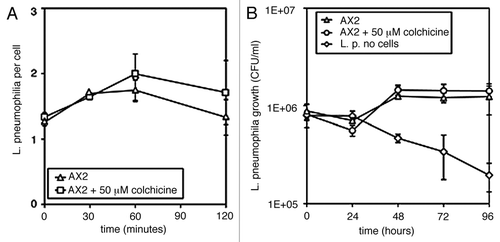
The major defect in the RacH-null mutant has been reported to be defective acidification of early endosomes.Citation9 Impaired acidification can explain the stimulatory effect on Legionella intracellular proliferation. LCV fusion with vesicles carrying V-H+ ATPase is inhibited by wild type Legionella, a process further enhanced by PI3K inactivation, resulting in increased Legionella proliferation.Citation1,Citation12 If LCV acidification in the RacH null mutant is impaired, Legionella proliferation is expected to be stimulated in comparison to the parental strain, similarly to what found for PI3K-null mutants. Macropinosome or phagosome acidification in Dictyostelium is mainly due to recruitment of the V-H+ ATPase from late endosomes.Citation13,Citation14 RacH could regulate traffic of acidic vesicles along actin filaments or microtubules. Following ingestion, the Legionella-containing vacuole is rapidly transported about the cell along microtubules, a process closely resembling phagosome or macropinosome transport.Citation15 We tested whether cell treatment with colchicineCitation16 would affect Legionella infection. To favor the depolymerizing effect of the drug, wild type AX2 cells were first incubated on ice for 15 min, the drug was then added and the cells were warmed up, before incubation with the bacteria. As shown in , neither uptake nor intracellular proliferation of Legionella were altered by the drug in comparison to control cells. Actin inhibitors, such as cytochalasin A or latrunculin A, on the other hand, inhibited uptake but not intracellular replication of Legionella.Citation1 In addition, Somesh et al.Citation9 reported that cell motility was unaffected in the mutant, ruling out major potential effects of RacH disruption on the actin cytoskeleton. Thus, how RacH inactivation alters vesicle acidification remains unclear.
Acidification regulates the activity of the iron transporter Nramp1. Both in Dictyostelium and macrophages, Nramp1 is located on endo-lysosomal membrane. Nramp1 is essential for iron transport across the phagosome or macropinosome membrane, and we have shown that Nramp1 pumps iron outside the vesicles by a proton gradient-driven mechanism,Citation17 as originally proposed by Gros and coworkers for macrophages.Citation18,Citation19 Iron promotes Legionella infection,Citation20 therefore recruitment of Nramp1, but not V-H+ ATPase, to the LCV generates a friendly environment for replication of Legionella. Accordingly, the bacteria proliferate better in Nramp1-null mutants, whereas Nramp1 constitutive overexpression inhibits Legionella proliferation;Citation17 this inhibition is reversed by the PI3K inhibitor LY294002.Citation1 This latter effect can be explained by the additional inhibitory effect of LY294002 on vacuolar ATPase recruitment to the LCV.Citation1
In addition to Legionella, intracellular multiplication of Mycobacteria (M. avium and M. marinum) was previously shown to also be enhanced in Dictyostelium RacH-nullCitation21 as well as Nramp1-null cells.Citation17 This suggests that neutralization of Nramp1, and possibly other divalent metal ion transporters,Citation21 in the replication vacuole via impairment of acidification is a common feature of both pathogens.
There are similarities and differences between Legionella and Mycobacterium infection. Hagedorn and SoldatiCitation21 reported transient acidification in a majority of M. marinum phagosomes during the first 90 min of infection, though afterwards the signal became undetectable. Acidification was strongly reduced in the RacH-null mutant. For L. pneumophila, we found that only a minority (10–15%) of the LCV's co-localized with V-H+ ATPase during the first 2 h post-infection,Citation1 consistent with previous data.Citation12 We did not quantify V-H+ ATPase recruitment in the RacH-null mutant during Legionella infection, though we can bona fide assume a value near zero, similarly to what found upon PI3K inhibition.
Recruitment of the post-lysosomal marker vacuolin, a member of the flotillin family, was strongly delayed both in L. pneumophilaCitation1 and M. marinumCitation21 infection, an indication that both pathogens interfere not only with acidification of the replication vacuole but also with its post-lysosomal traffic. Nevertheless, M. marinum proliferates and escapes the cell, probably via cell-to-cell transmission, after rupturing the replication vacuole and without extensive cell lysis.Citation22 In the case of Legionella we observed, instead, extensive cell rounding up and lysis after 24–48 h post-infection. Thus the results suggest that both pathogens avoid acidification, albeit with different modalities, and by so doing impair divalent metal ion depletion in the replication vacuole. Following proliferation M. marinum exploits an exocytic pathway to leave the cell, whereas L. pneumophila apparently induces cell lysis. Whether the acquisition in the LCV of post-lysosomal markers involved in exocytosis plays any functional role in the case of Legionella remains open.
Figures and Tables
Figure 1 Legionella pneumophila uptake and intracellular proliferation in the RacH-null mutant. (A) Bacterial uptake was measured by plating parental AX2 or RacH-null cells on 24-well tissue culture plates and adding L. pneumophila at a MOI of 10. To promote uptake, the bacteria were sedimented onto the cell layer by centrifugation. At the time indicated on the abscissa, extracellular bacteria were killed by short incubation with gentamycin and the number of L. pneumophila per cell was calculated by determining the colony forming units (CFU) per ml and normalizing for the cell number. (B) L. pneumophila intracellular growth in parental AX2 cells or RacH-null mutant was measured as above by using a MOI of 1 and by omitting gentamycin treatment. The CFU was determined at the time indicated in the abscissa. Under the conditions used, extracellular bacteria failed to grow.
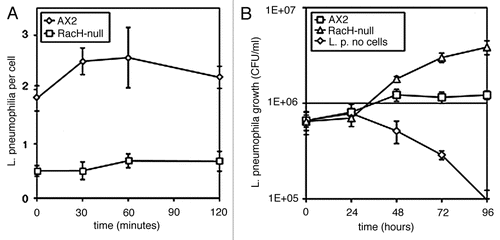
Figure 2 Legionella pneumophila uptake (A) and intracellular proliferation (B) in cells treated with the microtubule inhibitor colchicine. AX2 cells were preincubated on ice for 15 min, colchicine at 0.05 mM was added and the cells were warmed up to 25°C before addition of the bacteria. L. pneumophila uptake (A) and growth (B) were assessed as described in the legend to .
Acknowledgements
We thank Francisco Rivero for the RacH-null mutant. This work was supported by funds of the RSF Programme of the Piemonte Region, Italy.
Addendum to:
References
- Peracino B, Balest A, Bozzaro S. Phosphoinositides differentially regulate bacterial uptake and Nramp1-induced resistance to Legionella infection in Dictyostelium. J Cell Sci 2010; 123:4039 - 4051
- Seastone DJ, Zhang L, Buczynski G, Rebstein P, Weeks G, Spiegelman G, et al. The small Mr Ras-like GTPase Rap1 and the phospholipase C pathway act to regulate phagocytosis in Dictyostelium discoideum. Mol Biol Cell 1999; 10:393 - 406
- Peracino B, Borleis J, Jin T, Westphal M, Schwartz JM, Wu L, et al. G protein beta subunit-null mutants are impaired in phagocytosis and chemotaxis due to inappropriate regulation of the actin cytoskeleton. J Cell Biol 1998; 141:1529 - 1537
- Dormann D, Weijer G, Dowler S, Weijer CJ. In vivo analysis of 3-phosphoinositide dynamics during Dictyostelium phagocytosis and chemotaxis. J Cell Sci 2004; 117:6497 - 6509
- Zhou K, Pandol S, Bokoch G, Traynor-Kaplan AE. Disruption of Dictyostelium PI3K genes reduces [32P] phosphatidylinositol-3,4-bisphosphate and [32P]phosphatidylinositol trisphosphate levels, alters F-actin distribution and impairs pinocytosis. J Cell Sci 1998; 111:283 - 294
- Botelho RJ, Teruel M, Dierckman R, Anderson R, Wells A, York JD, et al. Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J Cell Biol 2000; 151:1353 - 1368
- Scott CC, Dobson W, Botelho RJ, Coady-Osberg N, Chavrier P, Knecht DA, et al. Phosphatidylinositol-4,5-bisphosphate hydrolysis directs actin remodeling during phagocytosis. J Cell Biol 2005; 169:139 - 149
- Bozzaro S, Bucci C, Steinert M. Phagocytosis and host-pathogen interactions in Dictyostelium with a look at macrophages. Int Rev Cell Mol Biol 2008; 271:253 - 300
- Somesh BP, Neffgen C, Iijima M, Devreotes P, Rivero F. Dictyostelium RacH regulates endocytic vesicular trafficking and is required for localization of vacuolin. Traffic 2006; 7:1194 - 1212
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 2005; 21:247 - 269
- Rivero F, Somesh BP. Signal transduction pathways regulated by Rho GTPases in Dictyostelium. J Muscle Res Cell Motil 2002; 23:737 - 749
- Weber SS, Ragaz C, Reus K, Nyfeler Y, Hilbi H. Legionella pneumophila exploits PI(4)P to anchor secreted effector proteins to the replicative vacuole. PLoS Pathog 2006; 2:46
- Clarke M, Kohler J, Arana Q, Liu T, Heuser J, Gerisch G. Dynamics of the vacuolar H(+)-ATPase in the contractile vacuole complex and the endosomal pathway of Dictyostelium cells. J Cell Sci 2002; 115:2893 - 2905
- Clarke M, Maddera L, Engel U, Gerisch G. Retrieval of the vacuolar H-ATPase from phagosomes revealed by live cell imaging. PLoS One 2010; 5:8585
- Lu H, Clarke M. Dynamic properties of Legionella-containing phagosomes in Dictyostelium amoebae. Cell Microbiol 2005; 7:995 - 1007
- Rubino S, Unger E, Fogu G, Cappuccinelli P. Effect of microtubule inhibitors on the tubulin system of Dictyostelium discoideum. Z Allg Mikrobiol 1982; 22:127 - 131
- Peracino B, Wagner C, Balest A, Balbo A, Pergolizzi B, Noegel AA, et al. Function and mechanism of action of Dictyostelium Nramp1 (Slc11a1) in bacterial infection. Traffic 2006; 7:22 - 38
- Forbes JR, Gros P. Divalent-metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends Microbiol 2001; 9:397 - 403
- Cellier MF, Courville P, Campion C. Nramp1 phagocyte intracellular metal withdrawal defense. Microbes Infect 2007; 9:1662 - 1670
- Robey M, Cianciotto NP. Legionella pneumophila feoAB promotes ferrous iron uptake and intracellular infection. Infect Immun 2002; 70:5659 - 5669
- Hagedorn M, Soldati T. Flotillin and RacH modulate the intracellular immunity of Dictyostelium to Mycobacterium marinum infection. Cell Microbiol 2007; 9:2716 - 2733
- Hagedorn M, Rohde KH, Russell DG, Soldati T. Infection by tubercular mycobacteria is spread by nonlytic ejection from their amoeba hosts. Science 2009; 323:1729 - 1733