Abstract
Astrocytes have been recently identified as important components of the tripartite synaptic complex. There is growing evidence that astrocytes regulate synaptic functions, in part, through the release of gliotransmitters. In a recent study, we have demonstrated that ephrinB3 could stimulate astrocytic release of D-serine through activation of EphB3 and EphA4 receptors. Eph receptors regulate this response by inducing the dephosphorylation of PKCa and activation of serine racemase to convert L-serine to D-serine. We now investigated whether ephrinB3 would increase the release of glutamine, which is also synthesized from serine and play important roles in regulating synaptic responses. Using HPLC, we observed an enhanced release of L/D-serine and glutamine from cultured astrocytes following ephrinB1 and/or ephrinB3 stimulation. In the absence of EphB3 and EphA4, ephrinB3-enhanced release of L/D-serine and glutamine was not observed. These studies provide evidence that Eph receptors may play broader roles in regulating gliotransmitter release from astrocytes, which could have important implications on synaptic transmission and learning and memory processes.
There is a close association between astrocytes and neurons in both spatial location and biological functions, although our understanding of the relevance of these interactions in learning and memory are largely unknown. Astrocytic membranes are often separated by as little as 20 nm from neuronal membranes,Citation1 and in synaptic regions, astrocytic processes wrap and ensheathe neuronal synapses.Citation1 Both neuronal- and glial-derived transmitters are believed to play important roles in a variety of responses; such as increasing excitability, modulation of neuronal transmission and synchronization of synaptic events.Citation2 However, the mechanisms that regulate the cross-talk between neurons and astrocytes are less clear. Recently, we have implicated ephrinB3 and Eph receptors in modulating astrocytic-neuronal communication by regulating the release of D-serine.Citation3 EphrinB3 and EphB3/EphA4 receptors are membrane-bound proteins that are found on both neurons and astrocytes, and ligand-receptor interactions can lead to bidirectional signaling.Citation4 Furthermore, gene-targeted knockout mice for ephrinB3 show deficits in longterm potentiation (LTP).Citation5 Unfortunately, these knockout studies are not cell type specific, and cannot differentiate between neuronal and astrocytic functions for ephrins and Eph receptors.
Previously, we have identified that ephrinB3 can activate EphB3 and/or EphA4 to increase D-serine release from astrocytes, which could have important implications in synaptic transmission (). In particular, post-synaptic ephrinB3 could interact with astrocytic EphB3 and EphA4 receptors to activate serine racemase (SR) and the conversion of L-serine to D-serine. We showed that activation of Eph receptors regulates D-serine levels by enhancing PICK1-SR interactions and reducing PKCα activity. It is less clear how PKCα acts in the reduction of D-serine levels but it is clear that activation of Eph receptors blocks its phosphorylation and activity. These findings are supported in gene-targeted knockout mice, where reduced D-serine release, PICK1-SR association and LTP were associated with the absence of EphB3 and EphA4.Citation3 However, cell-specific knockouts studies are required to identify whether astrocyte-derived Ephs are critical in mediating these biological responses.
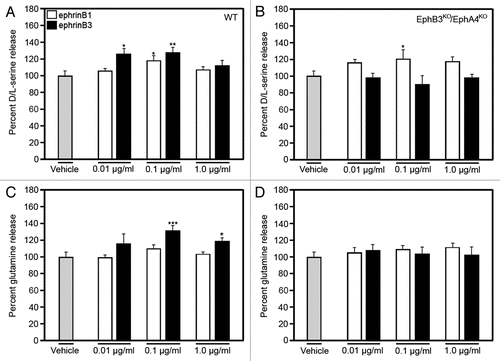
We might predict that these findings may not be unique to D-serine, and that amino acids, such as glutamine, may be also regulated by the interaction of ephrinB3 with EphB3 and/or EphA4. For that reason, we used high-performance liquid chromatography (HPLC) to measure the levels of glutamine as compared to D/L-serine in cultured astrocytes following ephrinB3 stimulation. shows that similar to our previous study,Citation3 D/L-serine levels were increased in the presence of either ephrinB1 or ephrinB3 in wild type astrocytes but only ephrinB1 enhanced D/L-serine release in astrocytes derived from EphB3/EphA4 knockout mice ( and B). The absence of ephrinB3 stimulation of D/L-serine release supports the specificity of EphB3 and/or EphA4 for that particular ligand. Examination of glutamine, an important precursor of the transmitter glutamate and shown to be released by astrocytes to enhance glutamate synthesis through phosphate-activated glutaminase (PAG) in neurons,Citation6–Citation8 showed that ephrinB3, but not ephrinB1, can stimulate glutamine release (). Like D/L-serine, ephrinB3 did not enhance glutamine release in the absence of EphB3 and EphA4 (). These findings suggest that ephrinB3 may have a more pronounced role in activating gliotransmitter release through activation of EphB3 and EphA4 than other ephrins.
The mechanism by which ephrinB3 enhances the release of glutamine is currently unknown. However, SR has been shown to play a role in the synthesis of these molecules. Specifically, SR is a bifunctional enzyme that can both convert L-serine to D-serineCitation9,Citation10 and produce pyruvate through α,β-elimination of water from L- and D-serine.Citation11 In the mitochondria, pyruvate decarboxylation leads to the production of ATP and diverse amino acids such as glutamine or glutamate.Citation11,Citation12 Additional studies are needed to determine whether ephrinB3 increases glutamine synthesis, and whether ephrinB3 enhances glutamate and ATP synthesis and release. Together, these findings provide further support for a more global role for ephrins and Eph receptors in regulating gliotransmitter levels through pre- and post-synaptic interactions with astrocytes.
Figures and Tables
Figure 1 Ephrins and Eph receptors function in tripartite communication between astrocytes and neurons in the CA1 hippocampus. Post-synaptic ephrinB3 can activate EphB3 and/or EphA4 in astrocytes to inhibit phosphorylation of PKC and enhance the association of SR with PICK1, which in turn enhances D-serine synthesis and release. D-serine release can regulate synaptic transmission and plasticity through activation of NMDA receptors on postsynaptic membranes. L-serine and D-serine can be converted to pyruvate through α,β-elimination of water to produce glutamine. Glutamine is taken up by neurons following astrocytic release, which can be converted to glutamate and GABA. Astrocytes also express ephrins that could potentially interact with pre- and/or post-synaptic Ephs to modulate synaptic functions. Not depicted on this schematic are studies that describe pre-synaptic ephrins interacting with post-synaptic EphB2 to modulate NMDAR functions,Citation13,Citation14 and astrocytic ephrinA3 interacting with post-synaptic EphA4.Citation15
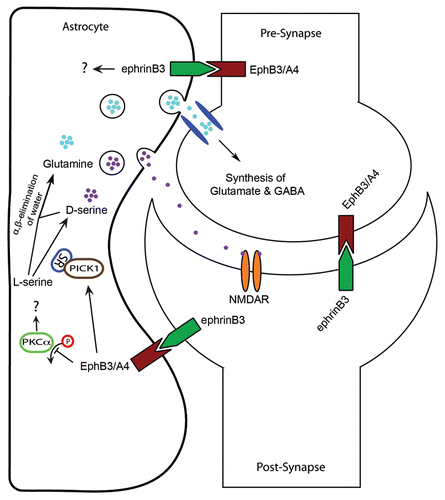
Figure 2 EphrinBs/Eph receptors signaling mediates D/L-serine and glutamine release from astrocytes. EphrinB1 stimulation increased L/D-serine and ephrinB3 stimulation increased D/L-serine and glutamine release from cultured astrocytes derived from wild-type mice 15 min after ephrinBs application (A and C). In the absence of EphB3 and EphA4 (EphB3KO/EphA4KO), ephrinB3 did not stimulate D/L-serine or glutamine release from cultured astrocytes. Amino acid levels were normalized against control amino acid level. Experiments performed in triplicate (n = 8–10). *p < 0.05; **p < 0.01; ***p < 0.001.
Acknowledgements
We would like to thank Drs. Mark Henkemeyer and Patrick Charnay for their generous gift of the mutant mice. This work was supported by the Miami Project to Cure Paralysis (D.J.L.), NIH/NINDS NS049545 and NS30291 (D.J.L.).
Addendum to:
References
- Porter JT, McCarthy KD. Astrocytic neurotransmitter receptors in situ and in vivo. Prog Neurobiol 1997; 51:439 - 455
- Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med 2007; 13:54 - 63
- Zhuang Z, Yang B, Theus MH, Sick JT, Bethea JR, Sick TJ, et al. EphrinBs Regulate D-Serine Synthesis and Release in Astrocytes. J Neurosci 2010; 30:16015 - 16024
- Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell 2008; 133:38 - 52
- Rodenas-Ruano A, Perez-Pinzon MA, Green EJ, Henkemeyer M, Liebl DJ. Distinct roles for ephrinB3 in the formation and function of hippocampal synapses. Dev Biol 2006; 292:34 - 45
- Shank RP, Aprison MH. Present status and significance of the glutamine cycle in neural tissues. Life Sci 1981; 28:837 - 842
- Kvamme E, Roberg B, Torgner IA. Phosphateactivated glutaminase and mitochondrial glutamine transport in the brain. Neurochem Res 2000; 25:1407 - 1419
- Albrecht J, Sonnewald U, Waagepetersen HS, Schousboe A. Glutamine in the central nervous system: function and dysfunction. Front Biosci 2007; 12:332 - 343
- Wolosker H, Blackshaw S, Snyder SH. Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc Natl Acad Sci USA 1999; 96:13409 - 13414
- Stevens ER, Esguerra M, Kim PM, Newman EA, Snyder SH, Zahs KR, et al. D-serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proc Natl Acad Sci USA 2003; 100:6789 - 6794
- Foltyn VN, Bendikov I, De Miranda J, Panizzutti R, Dumin E, Shleper M, et al. Serine racemase modulates intracellular D-serine levels through an alpha, beta-elimination activity. J Biol Chem 2005; 280:1754 - 1763
- Martineau M, Baux G, Mothet JP. D-serine signalling in the brain: friend and foe. Trends Neurosci 2006; 29:481 - 491
- Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, et al. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell 2000; 103:945 - 956
- Klein R. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat Neurosci 2009; 12:15 - 20
- Filosa A, Paixao S, Honsek SD, Carmona MA, Becker L, Feddersen B, et al. Neuron-glia communication via EphA4/ephrin-A3 modulates LTP through glial glutamate transport. Nat Neurosci 2009; 12:1285 - 1292