Abstract
Recent evidence indicates that the evolution of ultrasonic hearing in echolocating bats and cetaceans has involved adaptive amino acid replacements in the cochlear gene prestin. A substantial number of these changes have occurred in parallel in both groups, suggesting that particular amino acid residues might confer greater auditory sensitivity to high frequencies. Here we review some of these findings, and consider whether similar signatures of prestin protein sequence evolution also occur in mammals that possess high frequency hearing for passive localization, and, conversely, whether this gene has undergone less change in mammals that lack high frequency hearing.
Prestin in echolocating mammals
Prestin encodes a motor protein expressed in the outer hair cells (OHCs) of the basilar membrane (BM) in the cochlea, where it is thought to boost the BM's vibratory response to incoming sound waves.Citation1 It is this so-called “cochlear amplifier” that give mammals their remarkable auditory sensitivity as well as their narrow frequency tuning and dynamic range.Citation2 We and others have reported several lines of evidence that link substitutions in the cochlear gene prestin to the evolution of ultrasonic (>20 kHz) hearing in echolocating mammals. Briefly, unrelated lineages of echolocating bats,Citation3 and also bats and echolocating cetaceans,Citation4,Citation5 have undergone numerous parallel amino acid replacements in the prestin gene, some of which are common to both cases of convergence.Citation6 Strikingly, these replacements are sufficiently abundant to cause conflicts between the true species tree and phylogenetic reconstructions based on prestin, in which some echolocators are seen to group together in the same clade.Citation4,Citation5
Parallel substitutions could occur by chance, and conflicts between gene-tree and species-trees could reflect long-branch attraction or gene duplications, however, there is good evidence that convergence between bat and cetacean prestin has been driven by adaptive evolution. The strength of positive selection at sites along the dolphin prestin gene correlates with these sites' support for the grouping of dolphins with bats,Citation5 while bursts of positive selection have also been identified on the branch leading to bats with constant frequency echolocation (horseshoe and leaf-nosed bats),Citation3 the ancestral branch of all toothed whales and the ancestral branch of dolphins plus beaked whales.Citation6
Apart from particular sites under convergent and/or adaptive change, the overall level of protein sequence evolution appears to be linked to high hearing frequency. Within clades of bats and cetaceans, the number of amino acid substitutions counted along the evolutionary path leading to each taxon was found to correlate with its frequency of maximum auditory sensitivity, though this relationship disappears in bats after phylogenetic correction possibly due to a lack of taxonomic coverage.Citation6 Given all of these recent findings from echolocating taxa, it is tempting to speculate that there might be particular amino acid residues in prestin that confer greater auditory sensitivity to high frequencies in mammals.
Prestin in non-echolocating mammals
Echolocating bats and whales typically have the most sensitive hearing at high frequencies, using ultrasound for hunting, obstacle avoidance and orientation in space. Yet a much greater range of mammals can also perceive frequencies in the ultrasonic range.Citation7 Numerous small species including rodents, shrews and tree shrews, as well as larger taxa such as cats, seals, cows and dogs, are all known to be able to hear frequencies beyond the upper limit of human hearing.Citation8 Although these taxa mostly receive and process ultrasound solely for passive localization, some also produce ultrasonic vocalizations for communication,Citation9 and there is mounting evidence that a number of small insectivorous mammals can echolocate to some degreeCitation10 (although we classify these as non-echolocators here, to avoid confusion). At the other end of the spectrum, species such as human, non-human primates, elephants and sloths are generally considered to have poor high frequency hearing (e.g. ref. Citation8).
To test whether observed signatures of prestin evolution in echolocators also occur in other mammals with ultrasonic hearing, but to a lesser extent in mammals without ultrasonic hearing, we obtained and aligned all available mammal prestin sequences that contained over 90% of the coding region. This new alignment contained 51 sequences, including eleven new species from eight orders. An unconstrained Maximum-Likelihood phylogenetic tree based on this extended dataset recovered the previously reported monophyly of echolocating whales and bats, but contained no further instances of convergence or conflicts with the known species tree (figure not shown).
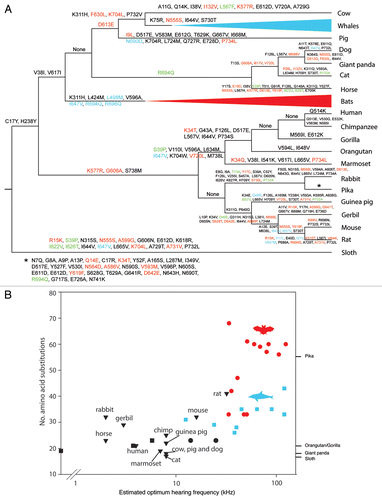
We derived prestin ancestral states for all nodes from the constrained species topology using the software CODEML, and traced substitutions leading to each taxon (). The number of amino acid substitutions varied widely among taxa, from just 13 in elephant to 59 in the pika. In the latter, more than 30 changes were inferred to have occurred since the split from the rabbit. High numbers of changes (>30) were also detected in all of the small rodents included (rat, mouse and gerbil), compared with both the primates (including human) that had around 22 changes each, and the other large mammals that had 23 each. Changes that were convergent with those seen in echolocators were distributed relatively evenly across all branches, and showed no obvious association with any particular taxa ().
A plot of counts of substitutions against corresponding estimated frequencies of optimal hearing revealed a significant positive relationship (R = 0.7, P < 0.0001). However, following phylogenetic correction by regressing pairwise differences in hearing frequency and substitutions, this trend was no longer significant (R = 0.04, NS, independent contrasts). Prestin sequences from more species are needed to determine whether this lack of effect is real, or reflects uneven taxonomic coverage. Regardless, it is clear that during their evolution echolocating bats and cetaceans have typically undergone many more changes in this gene than have Old World fruit bats, baleen whales and other non-echolocating mammals (). Of the five species for which no hearing data were available, the larger mammals showed similarly low numbers of changes as their sister taxa. On the other hand, the pika was a dramatic outlier, having undergone similar levels of change to echolocating bats, and thus making a potentially interesting candidate for an audiogram study.
Combining data from non-echolocating mammals to the results of our earlier studies of bats and dolphins adds some support to the idea that the tempo of change in Prestin correlates positively with the evolution of ultrasonic hearing in mammals. Alternative explanations for lineage-specific differences in molecular evolution due to variation in effective population sizesCitation11 are unlikely to account for higher numbers of substitutions in groups as diverse as bats, rodents and cetaceans. Yet the mechanism by which observed amino acid replacements in prestin might promote auditory sensitivity to high frequencies in echolocating and other taxa is not known. One possibility is that they result in conformational changes of the prestin protein, which in turn alter the shape and stiffness of the OHCs thereby allowing them to vibrate faster. For a given point along the BM, such upward tuning of OHC vibrations would allow closer matching to the frequencies of the ultrasonic echoes used by bats and cetaceans.Citation2 Alternatively, the prestin protein in echolocating mammals might be adapted to facilitate transmission of vibrations to neural excitation, a pathway that has been shown to be impaired in prestin -/- mice.Citation2
Figures and Tables
Figure 1 (A) Prestin amino acid substitutions mapped onto the evolutionary path for each taxon. Red amino acid substitutions are convergent with one or more lineages of echolocating bat, blue substitutions are convergent with one or more lineages of echolocating whale and green are convergent with both of these groups. Changes along the pika branch are shown by an asterisk. Names and sequence details of echolocating taxa, cow, pig, horse, dog, cat, human, rabbit, gerbil, mouse and rat are listed in ref. Citation6. Other mammal sequences were obtained from GenBank (giant panda XM_002928662, opossum XM_001371300 and platypus XM_001507913, with the latter two used as outgroups) or from Ensembl using BLAT. (B) Number of prestin amino acid substitutions versus estimated frequency of peak hearing sensitivity for mammals for which audiogram data were available (listed in ref. Citation6 and refs. Citation12—Citation24). Note that the elephant's hearing data is based on the Indian elephant, whereas the gene sequence is from the African elephant. Red and black circles represent echolocating and non-echolocating bats, respectively, and blue and black squares are echolocating and non echolocating baleen whales, respectively. Non-echolocating mammals are shown as black triangles. Species for which auditory data were not available are listed on the right-hand axis..
Acknowledgements
We thank Xinpu Yuan for help with data analysis.
Addendum to:
References
- Liberman MC, Gao JG, He DZZ, Wu XD, Jia SP, Zuo J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature 2002; 419:300 - 304
- Mellado Lagarde MM, Drexl M, Lukashkin AN, Zuo J, Russell IJ. Prestin's role in cochlear frequency tuning and transmission of mechanical responses to neural excitation. Curr Biol 2008; 18:200 - 202
- Li G, Wang JH, Rossiter SJ, Jones G, Cotton JA, Zhang SY. The hearing gene Prestin reunites echolocating bats. Proc Natl Acad Sci USA 2008; 105:13959 - 13964
- Li Y, Liu Z, Shi P, Zhang JZ. The hearing gene Prestin unites echolocating bats and whales. Curr Biol 2010; 20:R55 - R56
- Liu Y, Cotton JA, Shen B, Han XQ, Rossiter SJ, Zhang SY. Convergent sequence evolution between echolocating bats and dolphins. Curr Biol 2010; 20:R53 - R54
- Liu Y, Rossiter SJ, Han XQ, Cotton JA, Zhang SY. Cetaceans on a molecular fast track to ultrasonic hearing. Curr Biol 2010; 20:1834 - 1839
- Heffner HE, Heffner RS. Basbaum AI, Kaneko A, Shepherd GM, Westheimer G. High-Frequency Hearing. The Senses: A Comprehensive Reference 2008; 3:San Diego Academic Press 55 - 60
- Vater M, Kössl M. Comparative aspects of cochlear functional organization in mammals. Hear Res 2011; 273:1–2 89 - 99
- Awbrey FT, Thomas JA, Evans WE. Thomas JA, Moss CF, Vater M. Ultrasonic underwater sounds from a captive leopard seal (Hydrurga leptonyx). Echolocation in Bats and Dolphins 2003; Chicago & London University of Chicago Press 535 - 541
- Siemers BM, Schauermann G, Turni H, von Merten S. Why do shrews twitter? Communication or simple echo-based orientation. Biol Lett 2009; 5:593 - 596
- Woolfit M. Effective population size and the rate and pattern of nucleotide substitutions. Biol Lett 2009; 5:417 - 420
- Seiden HR. Auditory acuity of the marmoset monkey (Hapale jacchus) 1958; Princeton University Unpublished Doctoral Dissertation
- Kojima S. Comparison of auditory functions in the chimpanzee and human. Folia Primatol 1990; 55:62 - 72
- Jackson LL, Heffner RS, Heffner HE. Free-field audiogram of the Japanese macaque (Macaca fuscata). J Acoust Soc Am 1996; 106:3017 - 3023
- Heffner H, Masterton B. Hearing in glires: domestic rabbit, cotton rat, feral house mouse, and kangaroo rat. J Acoust Soc Am 1980; 68:1584 - 1599
- Ryan A. hearing sensitivity of the Mongolian gerbil, Meriones unguiculatis. J Acoust Soc Am 1976; 59:1222 - 1226
- Heffner HE, Heffner RS, Contos C, Ott T. Audiogram of the hooded Norway rat. Hear Res 1994; 73:244 - 247
- Koay G, Heffner RS, Heffner HE. Behavioral audiograms of homozygous medJ mutant mice with sodium channel deficiency and unaffected controls. Hear Res 2002; 171:111 - 118
- Heffner R, Heffner H, Masterton B. Behavioral measurements of absolute and frequency-difference thresholds in guinea pig. J Acoust Soc Am 1971; 49:1888 - 1895
- Heffner HE. Hearing in large and small dogs: Absolute thresholds and size of the tympanic membrane. Behav Neurosci 1983; 97:310 - 318
- Heffner RS, Heffner HE. Hearing range of the domestic cat. Hear Res 1985; 19:85 - 88
- Heffner RS, Heffner HE. Hearing in large mammals: The horses (Equus caballus) and cattle (Bos taurus). Behav Neurosci 1983; 97:299 - 309
- Heffner RS, Heffner HE. Hearing in domestic pigs (Sus scrofa) and goats (Capra hircus). Hear Res 1990; 48:231 - 240
- Heffner RS, Heffner HE. Hearing in the elephant (Elephas maximus): Absolute sensitivity, frequency discrimination, and sound localization. J Comp Physiol Psychol 1982; 96:926 - 944