Abstract
The shape of a neuron supplies valuable clues as to its function. Neurons typically extend a single long, thin axon, which will transmit signals, and several shorter and thicker dendrites, which will receive signals. The understanding of the means by which neurons acquire a polarized morphology is a fundamental issue in developmental neurobiology. The current view suggests that axon selection involves a stochastic mechanism. However, new data suggest that a polarized cytoplasm not only determines the position of neurite emergence, but also sets the conditions for morphological polarization. In vertebrates, neurons migrate before establishing their final morphology. Recent work shows that the polarized cytoplasm also determines how neurons migrate. Thus, neuronal migration might influence the processes by which neurons form an axon.
Neurons come in many shapes and sizes; in general, however, they maintain two domains: the axon and the somatodendritic compartment. It has become clear that the formation of these domains is the consequence of polarized differences in the subcellular mechanics of membrane delivery, actin dynamics and microtubule stability.Citation1–Citation4 It is not well understood, however, how the final positioning of these two domains is determined.
Axon Selection
The prevailing idea of the mechanisms underlying axon selection involves the stochastic selection of one of several, presumably equally competent, minor neurites by a competitive mechanism based on a contest between neurites that favors the establishment of the axonal fate (“tug of war” modelCitation5,Citation6). This concept is supported by the fact that axon formation can be directed by external factors when neurons are plated on to patterned substrates.Citation7
This hypothesis triggered a search for molecules that could act locally in a selected growth cone to initiate axonal outgrowth. Recently, several proteins that impose cellular asymmetry were identified to participate in axon growth, in part via signaling to the actin and microtubule cytoskeleton.Citation1,Citation2,Citation8 The question remains how these polarized molecules are concentrated in one neurite before, or during, the axon specification. Axon selection has been suggested to depend upon the translocation of a plus end motor protein (KIF5C), which belongs to the kinesin-1 superfamily.Citation9 This motor protein concentrates in one or two growth cones before axon formation, as well as in the established axon itself. KIF5C accumulation prior to axon formation is dynamic, and this protein constantly moves along neurites to reach the different neurite tips. Recent work supports the idea that there is a “radial movement” from the cell body to the neurite tip underlying the accumulation of KIF5C in the growth cone and that probably this and other motor proteins follow this principle to transport polarized signaling molecules to the axonal growth cone.Citation1,Citation2 In this regard, it was shown that molecules involved in axonal outgrowth, such as mPar3 and phosphatidylinositol-(3,4,5)-trisphosphate (PIP3), are polarized in developing neurons as a result of kinesin-mediated transport to the nascent axon tip.Citation10,Citation11 If it is the case that the kinesin family proteins are following this radial movement, it may be that the origin of this movement determines the position of axon outgrowth.
Centrosome Position Determines Neuronal Polarity
Recent data have shown that the site of axon formation is determined by cytoplasmic polarization;Citation12 the centrosome, Golgi apparatus and endosomes are clustered proximal to the site of the first neurite extension, and the axon has the highest probability of emerging from this location (Citation12). Moreover, polarized microtubule polymerization and membrane transport precede the formation of the primary neurite.Citation12 This is in accordance with observations that the centrosome and the Golgi are oriented towards the growing axon in cultured cerebellar granule neurons.Citation13 These findings challenge the concept that axonal fate is random, and suggest a cell-autonomous mechanism for the regulation of axon initiation.
The concept that axonal fate depends on centrosome position is consistent with established roles for the centrosome in neuronal differentiation and other biological processes, in which the centrosome is involved in defining cell polarity.Citation14 For instance, it was shown that, after fertilization, zygotes of C. elegans achieve polarization when the centrosome, provided by the sperm, is proximal to the embryo cortex and triggers the accumulation and segregation of the PAR proteins.Citation15 In this system, the centrosome is required to initiate, but not to maintain, polarity. Similarly, in cultured hippocampal neurons, it was shown that, when the axon is truncated to the length of the remaining neurites, another neurite could then become the axonCitation16 without the rotation of the centrosome to this new, alternative, axon.Citation17 This indicates that the centrosome is involved in initiating polarization and suggests that it confers to the first neurite a quantitative, rather than a qualitative, signal that provides this neurite with a growth advantage over the others.
Neurites appear in a sequential fashion, with the second neurite emerging opposite to the first.Citation17 This bipolar pattern of neurite differentiation underlies a bipolar organization of the cell that consists of more microtubules and membrane activity initially near the centrosome and, later, in the opposite pole.Citation17 This intrinsic bipolarity is maintained during development since, upon axotomy, the new axon formed with higher probability at the opposite pole of the original axon.Citation17 These data suggest that the polarity information represents the quantity of material that every neurite needs to receive in order to support a determined growth rate. When all neurites are forced to grow at the same rate, multiple axons are produced.Citation18
In vitro versus in vivo
The concept that intrinsic polarity pre-determines the site of axon outgrowth is supported by earlier in situ observations of differentiating grasshopper neurons in which Lefcort and Bentley (1989) found that the centrosome and Golgi apparatus are found at the site of the future axon.Citation19 In apparent contrast, however, in retinal ganglion cells and neurons from the cerebellum of the developing zebrafish embryo, centrosome position does not predict the site of axon emergence.Citation20,Citation21 Interestingly, in the retinal ganglion cells the centrosome and the Par3 protein are localized opposite to the site of axon elongation even when the external environment is disrupted. The behavior of those cells resembles the bipolar radial neuronal progenitors from the developing cortex with the centrosome in the apical process or endfeet radiating microtubules towards the basal process to control the nuclear movement throughout the cell cycle.Citation22 These findings are in agreement with the concept of an intrinsic mechanism underlying axon selection. However, fruit flies lacking centrioles develop a largely normal nervous system.Citation23
It remains to be elucidated whether, in neurons without centrioles, a polarized cytoplasm still underlies morphological differentiation. Along these lines, it was shown that the Golgi apparatus is a source of a large number of non-centrosomal microtubulesCitation24 that may compensate for a lack of centrioles. Moreover, some animal cells have centrosomes composed only of pericentriolar material with centrioles being undetectable.Citation25,Citation26 Despite a lack of centrioles, those cells are able to maintain an elaborate microtubule cytoskeleton.
Importantly, it was shown that the secreted UNC-6/netrin protein, which can attract or repel migrating cells, induces neuronal asymmetry and defines the site of axon formation early in the development of neurons of C. elegans.Citation27
These findings indicate that axon formation may have both conceptual and mechanistic similarities to the polarization that occurs during cell migration.Citation14 Changes in migration direction, following exposure to external stimuli, are associated with a re-orientation of the centrosome towards the leading edge.Citation14 Therefore, it is conceivable that an initial step of axon formation may occur when extracellular cues influence the orientation of the centrosome and polarized cytoplasm towards the future site of axon elongation.
Centrosome Motility is Essential for Proper Axon Formation in the Neocortex
In the developing cortex, the first signs of axon outgrowth in neurons that will end up in upper layers is evident in neurons located in the subventricular zone (SVZ) and intermediate zone (IZ)Citation28–Citation30 that display a multipolar morphology. The multipolar neuron represents a transitional stage between the newborn bipolar neurons that ascend from the ventricular zone (VZ) to the SVZ/IZ, and the bipolar neurons that migrate out of the IZ and into the cortical plate (CP),Citation30 elongating axons apically towards the VZ.
An interesting question that arises from these observations concerns the mechanisms by which the VZ-targeted apical axon is selected. Throughout migration, the centrosome is actively producing microtubules in a bipolar fashion, which propels the nucleus and exerts a pulling force toward the leading process.Citation31 Thus, it is conceivable that, to preferentially send microtubules and membrane to the neurite that will grow as an axon, the centrosome must cease to propel the nucleus. The transitional multipolar stage fits well with this concept, by having a reduced capacity for migration. Furthermore, it was shown that, in multipolar cells, the centrosome is not static, but instead undergoes translocation apically and helps to sustain the growth of the neurite that eventually become the axon.Citation32 A detailed imaging analysis of axon formation in these multi-polar neurons, using cortical slices, found a substantial amount of neurite growth from the apical pole only when the centrosome was positioned towards this neurite.Citation32 In these studies, cells eventually resumed migration, with the centrosome oriented towards the leading process, and the apical neurite acquired sufficient length to be considered the axon. In agreement with these findings, it was recently shown that, during neuronal migration, the continuous growth of microtubules from the perinuclear region into and through, the initial segment of the axon likely is responsible for sustaining axon elongation.Citation33 These findings suggest that the positioning of the apical centrosome and Golgi apparatus ensures the preferential delivery of material necessary for axon specification, whereas neuronal migration sustains axon elongation. In future studies, it will be important to determine how external cues regulate the centrosome motility prior to axon formation, as environmental influences are known to pattern the cortical efferent projections.Citation34
Figures and Tables
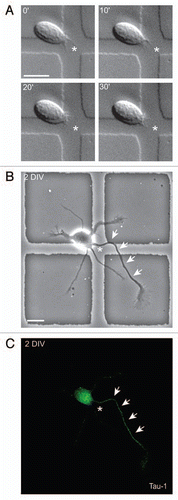
Figure 1 Axon selection can be predicted. (A) Time-lapse analysis of a freshly isolated rat hippocampal neuron. The cell has a dynamic sprout (asterisk). (B) The same cell as in (A), cultured for two days, formed an axon (arrows) from the place of the initial sprout (asterisk). (C) Tau-1 immunostaining confirmed the axonal identity of the neurite highlighted in (B). Time: minutes; scale bar: 10 mm.
Acknowledgements
F.C.A. is supported by a postdoctoral fellowship from the Simons Initiative on Autism and the Brain Infrastructure Grant Program. L.H.T. is an investigator of the Howard Hughes Medical Institute. We thank A. Mungenast and O. Durak for critical reading of the manuscript.
Addendum to:
References
- Wiggin GR, Fawcett JP, Pawson T. Polarity proteins in axon specification and synaptogenesis. Dev Cell 2005; 8:803 - 816
- Arimura N, Kaibuchi K. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat Rev Neurosci 2007; 8:194 - 205
- Witte H, Bradke F. The role of the cytoskeleton during neuronal polarization. Curr Opin Neurobiol 2008; 18:479 - 487
- Conde C, Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci 2009; 10:319 - 332
- Andersen SS, Bi GQ. Axon formation: a molecular model for the generation of neuronal polarity. Bioessays 2000; 22:172 - 179
- Bradke F, Dotti CG. Establishment of neuronal polarity: lessons from cultured hippocampal neurons. Curr Opin Neurobiol 2000; 10:574 - 581
- Esch T, Lemmon V, Banker G. Local presentation of substrate molecules directs axon specification by cultured hippocampal neurons. J Neurosci 1999; 19:6417 - 6426
- Polleux F, Snider W. Initiating and growing an axon. Cold Spring Harb Perspect Biol 2010; 2:1925
- Jacobson C, Schnapp B, Banker GA. A change in the selective translocation of the Kinesin-1 motor domain marks the initial specification of the axon. Neuron 2006; 49:797 - 804
- Shi SH, Cheng T, Jan LY, Jan YN. APC and GSK-3beta are involved in mPar3 targeting to the nascent axon and establishment of neuronal polarity. Curr Biol 2004; 14:2025 - 2032
- Horiguchi K, Hanada T, Fukui Y, Chishti AH. Transport of PIP3 by GAKIN, a kinesin-3 family protein, regulates neuronal cell polarity. J Cell Biol 2006; 174:425 - 436
- de Anda FC, Pollarolo G, Da Silva JS, Camoletto PG, Feiguin F, Dotti CG. Centrosome localization determines neuronal polarity. Nature 2005; 436:704 - 708
- Zmuda JF, Rivas RJ. The Golgi apparatus and the centrosome are localized to the sites of newly emerging axons in cerebellar granule neurons in vitro. Cell Motil Cytoskel 1998; 41:18 - 38
- Li R, Gundersen GG. Beyond polymer polarity: how the cytoskeleton builds a polarized cell. Nat Rev Mol Cell Biol 2008; 9:860 - 873
- Cowan CR, Hyman AA. Centrosomes direct cell polarity independently of microtubule assembly in C. elegans embryos. Nature 2004; 431:92 - 96
- Dotti CG, Banker GA. Experimentally induced alteration in the polarity of developing neurons. Nature 1987; 330:254 - 256
- Calderon de Anda F, Gartner A, Tsai LH, Dotti CG. Pyramidal neuron polarity axis is defined at the bipolar stage. J Cell Sci 2008; 121:178 - 185
- Bradke F, Dotti CG. The role of local actin instability in axon formation. Science 1999; 283:1931 - 1934
- Lefcort F, Bentley D. Organization of cytoskeletal elements and organelles preceding growth cone emergence from an identified neuron in situ. J Cell Biol 1989; 108:1737 - 1749
- Zolessi FR, Poggi L, Wilkinson CJ, Chien CB, Harris WA. Polarization and orientation of retinal ganglion cells in vivo. Neural Develop 2006; 1:2
- Distel M, Hocking JC, Volkmann K, Koster RW. The centrosome neither persistently leads migration nor determines the site of axonogenesis in migrating neurons in vivo. J Cell Biol 2010; 191:875 - 890
- Tsai JW, Lian WN, Kemal S, Kriegstein AR, Vallee RB. Kinesin 3 and cytoplasmic dynein mediate interkinetic nuclear migration in neural stem cells. Nat Neurosci 2010; 13:1463 - 1471
- Basto R, Lau J, Vinogradova T, Gardiol A, Woods CG, Khodjakov A, et al. Flies without centrioles. Cell 2006; 125:1375 - 1386
- Efimov A, Kharitonov A, Efimova N, Loncarek J, Miller PM, Andreyeva N, et al. Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans Golgi network. Dev Cell 2007; 12:917 - 930
- Szollosi D, Calarco P, Donahue RP. Absence of centrioles in the first and second meiotic spindles of mouse oocytes. J Cell Sci 1972; 11:521 - 541
- Schatten H, Schatten G, Mazia D, Balczon R, Simerly C. Behavior of centrosomes during fertilization and cell division in mouse oocytes and in sea urchin eggs. Proc Natl Acad Sci USA 1986; 83:105 - 109
- Adler CE, Fetter RD, Bargmann CI. UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation. Nat Neurosci 2006; 9:511 - 518
- Shoukimas GM, Hinds JW. The development of the cerebral cortex in the embryonic mouse: an electron microscopic serial section analysis. J Comp Neurol 1978; 179:795 - 830
- Hatanaka Y, Murakami F. In vitro analysis of the origin, migratory behavior and maturation of cortical pyramidal cells. J Comp Neurol 2002; 454:1 - 14
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci 2004; 7:136 - 144
- Tsai LH, Gleeson JG. Nucleokinesis in neuronal migration. Neuron 2005; 46:383 - 388
- de Anda FC, Meletis K, Ge X, Rei D, Tsai LH. Centrosome motility is essential for initial axon formation in the neocortex. J Neurosci 2010; 30:10391 - 10406
- Tsai JW, Bremner KH, Vallee RB. Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nat Neurosci 2007; 10:970 - 979
- Polleux F, Giger RJ, Ginty DD, Kolodkin AL, Ghosh A. Patterning of cortical efferent projections by semaphorin-neuropilin interactions. Science 1998; 282:1904 - 1906