Abstract
The velvet belly lantern shark (Etmopterus spinax) is a common deep-sea shark that has been used, in the recent years, as a model for experimental studies on physiological control of shark luminescence. These studies demonstrated that, unlike any other luminous organism, the luminescence of this shark was under a dual control of hormones and neurotransmitters (or neuromodulators). The present paper, by making a short review of histological and pharmacological results from these studies, aims to propose a first model of luminescence control in E. spinax.
The velvet belly lantern shark (Etmopterus spinax) is a common deep-sea shark that possess the amazing capability to emit a visible light from thousands of tiny epidermal photogenic organs called photophores, which are made of a cluster of photogenic cells called photocytes sheated in a pigmented layer and topped by pigmented and lens cells ().Citation1 The organization of these organs as well as the physical characteristics of their light emission strongly suggest that they are involved in varied behaviors including antipredatory responseCitation2–Citation5 and intraspecific communication.Citation3,Citation6
To be ecologically successful, however, this bioluminescence needs to be properly controlled. In the past four years several experimental studies investigated the control of luminescence emitted by the photophores of E. spinax.Citation6–Citation10 They ended up on the amazing conclusion that these photogenic structures were actually controlled by two different types of substances: hormonesCitation6–Citation8 and neurotransmitters (or neuromodulators),Citation9,Citation10 contrary to all other intrinsically luminous organisms (i.e., organisms that produced light without the help of bacterial symbionts) known to date, in which the physiological control was exclusively nervous (). The present review aimed to synthesize the different pharmacological and histological results found to date in order to propose a first model of the photophore luminescence control in E. spinax.
Resting State
Recent pharmacological and immunohistochemical investigations demonstrated that an inhibitory GABA tonus exists in the photophores, which prevents undesired light emission from these photogenic organs by provoking pigment dispersion in pigmented cells recovering the photocytes (, part 1).Citation10 It was indeed suggested that photocytes are permanently stimulated by low levels of circulatory excitatory hormones, i.e., MT and PRL, hence would induce an undesired permanent glow from the shark's body without the presence of this inhibitory mechanism.
In addition, the same study shows the effects of this neurotransmitter to be mediated by the receptor GABAA, since application of the GABAA antagonist bicuculline, suppressing partially the effect of the inhibitory GABA tonus, provoked a weak light emission from the photophores ().Citation10 Interestingly, in the luminous worm Chaetopterus variopedatus, GABA also has a GABAA-mediated inhibitory effect on luminescence, while it demonstrates a GABAB-mediated stimulatory action on the luminescence of the brittle star Ophionereis fasciata.Citation11,Citation12
Luminescence Switch On
The light switch on in E. spinax photophores is induced by two hormones, which are also involved in elasmobranch physiological control of color change: (1) the MT, which is produced by the pineal gland and (2) the PRL, which is produced by the pars distalis of the pituitary gland (, part 2).Citation7 The light kinetics of these two hormones are, however, different: MT induces a slowly increasing long lasting (up to several hours) glow while PRL induces a quicker glow, which generally reaches a peak after 20 min and ends up within 1 h.Citation7 It has been suggested that this differential light course reflects a differential use of these hormones: MT would be especially involved in counterillumination, i.e., light production by an animal to obliterate its silhouette from below, while PRL would be involved in more periodic behaviors such as cohesive swimming/hunting and/or sexual communication.Citation6,Citation7
Although no immunohistochemistry has been performed to highlight the exact position of their receptors, it has been suggested that MT and PRL have the same targets in the photophores, although using different intracellular pathways: (1) the photocytes that they stimulate to glow and (2) the overlying pigmented cells in which they provoke pigment dispersion.Citation8,Citation10 Since both MT1/MT2 antagonist luzindole and MT2 antagonist 4P-PDOT inhibit MT-induced luminescence, this hormone probably acts through MT2 receptor, which appears to be negatively coupled to cyclic AMP ().Citation7 PRL, on its side, probably acts through a “shark PRLR (PRL receptor)” although this has not been tested, due to the current absence of shark PRLR antagonist in the distribution. Intracellular effects of PRL appears to be mediated by the JAK2 since luminescence induced by PRL is strongly inhibited by a JAK2 inhibitor ().Citation7
Luminescence Modulation
Nitric oxide (NO) has been recently demonstrated to consist in an additional control mechanism, probably responsible of the differential sensitivity to hormones in adult inviduals of E. spinax, according to the sex of the shark or to the part of the luminous pattern tested.Citation6,Citation9 Although NO does not have any effect per se on the photophore luminescence of the shark E. spinax, this versatile substance fastens the luminous response to MT and decreases the amplitude of PRL-induced luminescence probably by acting at the level of the photocytes since numerous NO synthases have been found in these photogenic cells (, part 3).Citation9 The effect of NO on PRL-induced luminescence are similar to those observed by NO on adrenalin-induced luminescence of the teleost Argyropelecus hemigymnus, where it is supposed to allow precise adjustments of light intensity for a convenient counterillumination.Citation13 It is likely that NO also functions as a fine tuner of E. spinax luminescence in counterilluminating behavior, but probably also in intraspecific functions.Citation9
NO is a freely diffusible substance which can exert its actions either through the production of GMPc or via the inhibition of mitochondrial respiration in the target cell. In E. spinax photophores, effects of NO on hormonally induced luminescence are probably mediated by cGMP, since this substance mimicks the effects of NO in these photogenic organs ().Citation9 Interestingly, only the cGMP-independent pathway was found in other luminous organisms using NO in control of their bioluminescence i.e., A. hemigymnus, the krill Meganyctiphanes norvegica and the fireflies Photuris sp.Citation13,Citation15,Citation16
Luminescence Switch Off
The luminescence switch off in E. spinax photophores is induced by the α-melanocyte stimulating hormone (α-MSH), a hormone also involved in the elasmobranch skin coloration control (, part 4).Citation7,Citation17 Although this has never been experimentally demonstrated, it is likely that α-MSH switches off the light by provoking pigment dispersion in pigmented cells topping the photocytes, similarly to its action on melanophores which provoke skin darkening, probably through a fixation to MC1 receptor ().Citation17
In addition, one cannot exclude GABA to be produced on demand to allow a quicker switch off the light from the photophores of this shark, but this remains to be tested.Citation10
Conclusions
The luminescence control mechanism of E. spinax is complex, involving different substances acting on different targets, and therefore allows a precise tuning of the light emission that certainly reflects the ecological importance of this latest in the life of this shark. Interestingly, it involves physiological pathways that are not found elsewhere in other luminous organisms, which illustrates the diversity of the luminescence phenomenon and its numerous independant appearances during the course of evolution.Citation18 Finally, it also gives clues to evolutionnary pathway of this capability in sharks, suggesting that the photophore control mechanism of these fishes originally evolved from its physiological control of color change, which involves the same hormones.
Abbreviations
| PRL | = | prolactin |
| MT | = | melatonin |
| E. spinax | = | Etmopterus spinax |
| NO | = | nitric oxide |
Figures and Tables
Figure 1 Model of photophore luminescence control in the shark Etmopterus spinax. (A) Luminescence control pathways present in a photophore (transversal section). Colored arrows indicate the targets of the different substances involved in the control of E. spinax's photogenesis. Symbols in color circles indicate the effect of these substances on luminescence: +, activatory; −, inhibitory; ±, modulatory. (B) Different luminescence states in a group of photophores (transversal section): (1) resting state—photocytes are weekly stimulated to glow by low levels of circulating hormones (MT and PRL; red arrows) while GABA (green arrows) prevent light to be emitted outside the photophores by provoking pigment expansion in the pigmented cells topping the photocytes; (2) luminescence switch on—high levels of circulating hormones (MT and PRL; red arrows) stimulate the photocytes to glow and provoke pigment retraction in pigmented cells topping the photocytes, counterbalancing the effect of GABA; (3) luminescence modulation—NO (blue arrows) modulate the effects of stimulatory hormones, probably by acting directly on the photocytes; (4) luminescence switch off—α-MSH (mauve arrows) inhibits the hormonally induced light, probably by acting on the pigmented cells topping the photocytes. α-MSH, α-melanocyte stimulating hormone; bs, blood sinus; CT, connective tissue; E, epidermis; GABA, γ-amino butyric acid; l, lens cell; MT, melatonin; NO, nitric oxide; p, pigmented cell; ph, photocyte; PL, pigmented layer; PRL, prolactin; ps, pigmented sheath.
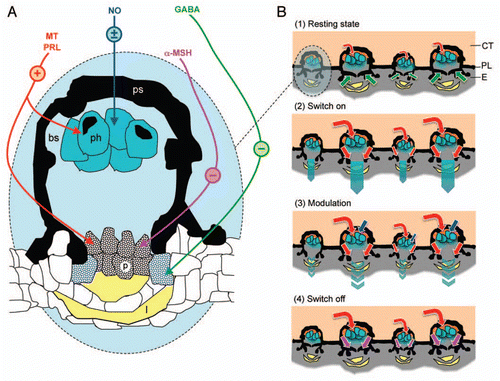
Table 1 Intrinsic pathways of substances involved in the luminescence control of Etmopterus spinax
Acknowledgements
J.M. Claes is a postdoctoral researcher of FRS-F.N.R.S. J. Mallefet is a research associate of FRS-F.N.R.S. Contribution to the Biodiversity Research Center (BDIV) and to the Centre Interuniversitaire de Biologie Marine (CIBIM).
References
- Hickling CF. The luminescence of the dogfish Spinax Niger. Nature, London 1928; 121:280 - 281
- Claes JM, Mallefet J. Early development of bioluminescence suggests camouflage by counter-illumination in the velvet belly lantern shark Etmopterus spinax (Squaloidea: Etmopteridae). J Fish Biol 2008; 73:1337 - 1350
- Claes JM, Mallefet J. Ontogeny of photophore pattern in the velvet belly lantern shark, Etmopterus spinax. Zoology 2009; 112:433 - 441
- Claes JM, Aksnes DL, Mallefet J. Phantom hunter of the fjords: Camouflage by counterillumination in a shark (Etmopterus spinax). J Exp Mar Biol Ecol 2010; 388:28 - 32
- Claes JM. Function and control of luminescence from lantern shark (Etmopterus spinax) photophores 2010; 232 Université catholique de Louvain Ph.D. thesis
- Claes JM, Mallefet J. Functional physiology of lantern shark (Etmopterus spinax) luminescent pattern: differential hormonal regulation of luminous zones. J Exp Biol 2010; 213:1852 - 1858
- Claes JM, Mallefet J. Hormonal control of luminescence from lantern shark (Etmopterus spinax) photophores. J Exp Biol 2009; 212:3684 - 3692
- Claes JM, Mallefet J. The lantern shark's light switch: turning shallow water crypsis into midwater camouflage. Biol Lett 2010; 6:685 - 687
- Claes JM, Krönström J, Holmgren S, Mallefet J. Nitric oxide in control of luminescence from lantern shark (Etmopterus spinax) photophores. J Exp Biol 2010; 213:3005 - 3011
- Claes JM, Krönström J, Holmgren S, Mallefet J. GABA inhibition of luminescence from lantern shark (Etmopterus spinax) photophores. Comp Biochem Physiol C 2011; 153:231 - 236
- Anctil M. Luminescence control in isolated notopods of the tube-worm Chaetopterus variopedatus: effects of cholinergic and GABAergic drugs. Comp Biochem Physiol C 1981; 68:187 - 194
- Mallefet J, Barker M, Byrne M, O'Hara T. Heinzeller T, Nebelsick JH. First study of bioluminescence in Ophionereis. Echinoderms: München 2004; London Taylor and Francis Group (Balkema) 299 - 304
- Krönström J, Holmgren S, Baguet F, Salpietro L, Mallefet J. Nitric oxide in control of luminescence from hatchetfish (Argyropelecus hemigymnus) photophores. J Exp Biol 2005; 208:2951 - 2961
- Jacklet JW. Nitric oxide signalling in invertebrates. Invert Neurosci 1997; 3:1 - 14
- Krönström J, Dupont S, Mallefet J, Thorndyke M, Holmgren S. Serotonin and nitric oxide interaction in the control of bioluminescence in northern krill, Meganyctiphanes norvegica (M. Sars). J Exp Biol 2007; 209:3179 - 3187
- Trimmer BA, Aprille JR, Dudzinski DM, Lagace CJ, Lewis SM, Miche T, et al. Nitric oxide and the control of firefly flashing. Science 2001; 292:2486 - 2488
- Gelsleichter J. Carrier J, Musik J, Heithaus M. Hormonal regulation of elasmobranch physiology. Biology of Sharks and their Relatives 2004; Boca Raton, FL CRC Press 287 - 293
- Hastings JW. Biological diversity, chemical mechanisms and evolutionnary origins of bioluminescent systems. J Mol Evol 1983; 19:309 - 321